Vocal premotor activity in the superior colliculus
- PMID: 17202477
- PMCID: PMC6672295
- DOI: 10.1523/JNEUROSCI.2683-06.2007
Vocal premotor activity in the superior colliculus
Abstract
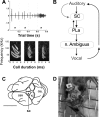
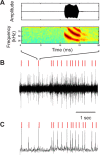
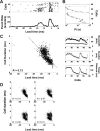
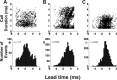
Chronic neural recordings were taken from the midbrain superior colliculus (SC) of echolocating bats while they were engaged in one of two distinct behavioral tasks: virtual target amplitude discrimination (VTAD) and real oscillating target tracking (ROTT). In the VTAD task, bats used a limited range of sonar call features to discriminate the amplitude category of echoes, whereas in the ROTT task, the bat produced dynamically modulated sonar calls to track a moving target. Newly developed methods for chronic recordings in unrestrained, behaving bats reveal two consistent bouts of SC neural activity preceding the onset of sonar vocalizations in both tasks. A short lead bout occurs tightly coupled to vocal onset (VTAD, -5.1 to -2.2 ms range, -3.6 +/- 0.7 ms mean lead time; ROTT, -3.0 to + 0.4 ms range, -1.2 +/- 1.3 ms mean lead time), and this activity may play a role in marking the time of each sonar emission. A long lead bout in SC activity occurs earlier and spreads over a longer interval (VTAD, -40.6 to -8.4 ms range, -22.2 +/- 3.9 ms mean lead time; ROTT, -29.8 to -7.1 ms range, -17.5 +/- 9.1 ms mean lead time) when compared with short lead events. In the goal-directed ROTT task, the timing of long lead event times vary with the bat's sonar call duration. This finding, along with behavioral studies demonstrating that bats adjust sonar call duration as they track targets at changing distance, suggests the bat SC contributes to range-dependent adjustments of sonar call duration.
Figures






Similar articles
-
Functional Organization and Dynamic Activity in the Superior Colliculus of the Echolocating Bat, Eptesicus fuscus.J Neurosci. 2018 Jan 3;38(1):245-256. doi: 10.1523/JNEUROSCI.1775-17.2017. Epub 2017 Nov 27. J Neurosci. 2018. PMID: 29180610 Free PMC article.
-
Natural echolocation sequences evoke echo-delay selectivity in the auditory midbrain of the FM bat, Eptesicus fuscus.J Neurophysiol. 2018 Sep 1;120(3):1323-1339. doi: 10.1152/jn.00160.2018. Epub 2018 Jun 20. J Neurophysiol. 2018. PMID: 29924708
-
Adaptive sonar call timing supports target tracking in echolocating bats.J Exp Biol. 2018 Sep 17;221(Pt 18):jeb176537. doi: 10.1242/jeb.176537. J Exp Biol. 2018. PMID: 29997156
-
Representation of perceptual dimensions of insect prey during terminal pursuit by echolocating bats.Biol Bull. 1996 Aug;191(1):109-21. doi: 10.2307/1543071. Biol Bull. 1996. PMID: 8776847 Review.
-
Neural processing of target distance by echolocating bats: functional roles of the auditory midbrain.Neurosci Biobehav Rev. 2011 Nov;35(10):2073-83. doi: 10.1016/j.neubiorev.2010.12.015. Epub 2011 Jan 14. Neurosci Biobehav Rev. 2011. PMID: 21238485 Free PMC article. Review.
Cited by
-
Motor functions of the superior colliculus.Annu Rev Neurosci. 2011;34:205-31. doi: 10.1146/annurev-neuro-061010-113728. Annu Rev Neurosci. 2011. PMID: 21456962 Free PMC article. Review.
-
Echolocating bats rely on audiovocal feedback to adapt sonar signal design.Proc Natl Acad Sci U S A. 2017 Oct 10;114(41):10978-10983. doi: 10.1073/pnas.1711892114. Epub 2017 Sep 25. Proc Natl Acad Sci U S A. 2017. PMID: 28973851 Free PMC article.
-
Wireless multi-channel single unit recording in freely moving and vocalizing primates.J Neurosci Methods. 2012 Jan 15;203(1):28-40. doi: 10.1016/j.jneumeth.2011.09.004. Epub 2011 Sep 12. J Neurosci Methods. 2012. PMID: 21933683 Free PMC article.
-
Orienting our view of the superior colliculus: specializations and general functions.Curr Opin Neurobiol. 2021 Dec;71:119-126. doi: 10.1016/j.conb.2021.10.005. Epub 2021 Nov 23. Curr Opin Neurobiol. 2021. PMID: 34826675 Free PMC article. Review.
-
Regulation of bat echolocation pulse acoustics by striatal dopamine.J Exp Biol. 2011 Oct 1;214(Pt 19):3238-47. doi: 10.1242/jeb.058149. J Exp Biol. 2011. PMID: 21900471 Free PMC article.
References
-
- Bankman IN, Johnson KO, Schneider W. Optimal detection, classification, and superposition resolution in neural waveform recordings. IEEE Trans Biomed Eng. 1993;40:836–841. - PubMed
-
- Casseday JH, Kobler JB, Isbey SF, Covey E. Central acoustic tract in an echolocating bat: an extralemniscal auditory pathway to the thalamus. J Comp Neurol. 1989;287:247–259. - PubMed
-
- Chaturvedi V, Van Gisbergen JA. Perturbation of combined saccade-vergence movements by microstimulation in monkey superior colliculus. J Neurophysiol. 1999;81:2279–2296. - PubMed
-
- Chaturvedi V, Van Gisbergen JA. Stimulation in the rostral pole of monkey superior colliculus: effects on vergence eye movements. Exp Brain Res. 2000;132:72–78. - PubMed
-
- Covey E, Hall WC, Kobler JB. Subcortical connections of the superior colliculus in the mustache bat, Pteronotus parnellii. J Comp Neurol. 1987;263:179–197. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
