Disynaptic amplification of metabotropic glutamate receptor 1 responses in the olfactory bulb
- PMID: 17202480
- PMCID: PMC6672277
- DOI: 10.1523/JNEUROSCI.2439-06.2007
Disynaptic amplification of metabotropic glutamate receptor 1 responses in the olfactory bulb
Abstract
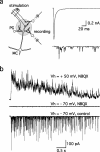
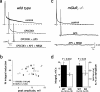
Sensory systems often respond to rapid stimuli with high frequency and fidelity, as perhaps best exemplified in the auditory system. Fast synaptic responses are fundamental requirements to achieve this task. The importance of speed is less clear in the olfactory system. Moreover, olfactory bulb output mitral cells respond to a single stimulation of the sensory afferents with unusually long EPSPs, lasting several seconds. We examined the temporal characteristics, developmental regulation, and the mechanism generating these responses in mouse olfactory bulb slices. The slow EPSP appeared at postnatal days 10-11 and was mediated by metabotropic glutamate receptor 1 (mGluR1) and NMDA receptors. mGluR1 contribution was unexpected because its activation usually requires strong, high-frequency stimulation of inputs. However, dendritic release of glutamate from the intraglomerular network caused spillover-mediated recurrent activation of metabotropic glutamate receptors. We suggest that persistent responses in mitral cells amplify the incoming sensory information and, along with asynchronous inputs, drive odor-evoked slow temporal activity in the bulb.
Figures

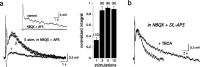



Similar articles
-
Distinct temporal filters in mitral cells and external tufted cells of the olfactory bulb.J Physiol. 2017 Oct 1;595(19):6349-6362. doi: 10.1113/JP274608. J Physiol. 2017. PMID: 28791713 Free PMC article.
-
Presynaptic metabotropic glutamate receptors in adult and developing neurons: autoexcitation in the olfactory bulb.J Comp Neurol. 1995 Aug 21;359(2):253-71. doi: 10.1002/cne.903590206. J Comp Neurol. 1995. PMID: 7499528
-
Glutamatergic transmission and plasticity between olfactory bulb mitral cells.J Physiol. 2008 Apr 15;586(8):2107-19. doi: 10.1113/jphysiol.2007.149575. Epub 2008 Feb 14. J Physiol. 2008. PMID: 18276730 Free PMC article.
-
[Metabotropic glutamate receptor: its ligands and function in the olfactory system].Nihon Yakurigaku Zasshi. 1999 Feb;113(2):73-83. doi: 10.1254/fpj.113.73. Nihon Yakurigaku Zasshi. 1999. PMID: 10205782 Review. Japanese.
-
Synaptic transmission and modulation in the olfactory bulb.Curr Opin Neurobiol. 1993 Aug;3(4):540-7. doi: 10.1016/0959-4388(93)90053-2. Curr Opin Neurobiol. 1993. PMID: 8219719 Review.
Cited by
-
Bulbar microcircuit model predicts connectivity and roles of interneurons in odor coding.PLoS One. 2015 May 5;10(5):e0098045. doi: 10.1371/journal.pone.0098045. eCollection 2015. PLoS One. 2015. PMID: 25942312 Free PMC article.
-
Inhibitory circuits of the mammalian main olfactory bulb.J Neurophysiol. 2017 Oct 1;118(4):2034-2051. doi: 10.1152/jn.00109.2017. Epub 2017 Jul 19. J Neurophysiol. 2017. PMID: 28724776 Free PMC article. Review.
-
Cellular and Synaptic Mechanisms That Differentiate Mitral Cells and Superficial Tufted Cells Into Parallel Output Channels in the Olfactory Bulb.Front Cell Neurosci. 2020 Dec 22;14:614377. doi: 10.3389/fncel.2020.614377. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33414707 Free PMC article.
-
Experience-dependent modification of primary sensory synapses in the mammalian olfactory bulb.J Neurosci. 2007 Aug 29;27(35):9427-38. doi: 10.1523/JNEUROSCI.0664-07.2007. J Neurosci. 2007. PMID: 17728456 Free PMC article.
-
Loss of olfactory cell adhesion molecule reduces the synchrony of mitral cell activity in olfactory glomeruli.J Physiol. 2011 Apr 15;589(Pt 8):1927-41. doi: 10.1113/jphysiol.2011.206276. Epub 2011 Feb 21. J Physiol. 2011. PMID: 21486802 Free PMC article.
References
-
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. - PubMed
-
- Ache BW, Young JM. Olfaction: diverse species, conserved principles. Neuron. 2005;48:417–430. - PubMed
-
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. - PubMed
-
- Aroniadou-Anderjaska V, Ennis M, Shipley MT. Glomerular synaptic responses to olfactory nerve input in rat olfactory bulb slices. Neuroscience. 1997;79:425–434. - PubMed
-
- Axel R. Scents and sensibility: a molecular logic of olfactory perception (Nobel lecture) Angew Chem Int Ed Engl. 2005;44:6110–6127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
