Depolarization and neurotransmitter regulation of vasopressin gene expression in the rat suprachiasmatic nucleus in vitro
- PMID: 17202481
- PMCID: PMC6672276
- DOI: 10.1523/JNEUROSCI.3739-06.2007
Depolarization and neurotransmitter regulation of vasopressin gene expression in the rat suprachiasmatic nucleus in vitro
Abstract
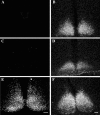
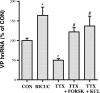
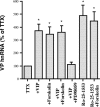
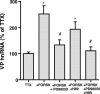
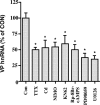
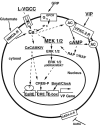
Vasopressin (VP) transcription in the rat suprachiasmatic nucleus (SCN) in organotypic culture was studied by in situ hybridization histochemistry using an intron-specific VP heteronuclear RNA probe. The circadian peak of VP gene transcription in the SCN in vitro is completely blocked by a 2 h exposure to tetrodotoxin (TTX) in the culture medium, and this TTX inhibition of VP gene transcription is reversed by exposure of the SCN to either forskolin or potassium depolarization. This suggests that an intrinsic, spontaneously active neuronal mechanism in the SCN is responsible for the cAMP- and depolarization-dependent pathways involved in maintaining peak VP gene transcription. In this paper, we evaluate a variety of neurotransmitter candidates, membrane receptors, and signal-transduction cascades that might constitute the mechanisms responsible for the peak of VP gene transcription. We find that vasoactive intestinal peptide (VIP) and a VPAC2 (VIP receptor subtype 2) receptor-specific agonist, Ro-25-1553, are the most effective ligands tested in evoking a cAMP-mitogen-activated protein kinase signal transduction cascade leading to an increase in VP gene transcription in the SCN. In addition, a second independent pathway involving depolarization activating L-type voltage-gated calcium channels and a Ca-dependent kinase pathway [inhibited by KN62 (1-[N,O-bis(5-isoquinolinesulphonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine)] rescues VP gene transcription in the presence of TTX. In the absence of TTX, these independent pathways appear to act in a cooperative manner to generate the circadian peak of VP gene transcription in the SCN.
Figures





Similar articles
-
Effects of VPAC2 receptor activation on membrane excitability and GABAergic transmission in subparaventricular zone neurons targeted by suprachiasmatic nucleus.J Neurophysiol. 2009 Sep;102(3):1834-42. doi: 10.1152/jn.91261.2008. Epub 2009 Jul 1. J Neurophysiol. 2009. PMID: 19571188
-
Neuronal activity is required for the circadian rhythm of vasopressin gene transcription in the suprachiasmatic nucleus in vitro.Endocrinology. 2002 Nov;143(11):4165-71. doi: 10.1210/en.2002-220393. Endocrinology. 2002. PMID: 12399408
-
Sex-specific differences in the circadian pattern of action potential firing by rat suprachiasmatic nucleus vasopressin neurons.J Neuroendocrinol. 2023 Sep;35(9):e13273. doi: 10.1111/jne.13273. Epub 2023 May 3. J Neuroendocrinol. 2023. PMID: 37132408
-
Vasopressin neurotransmission and the control of circadian rhythms in the suprachiasmatic nucleus.Prog Brain Res. 1998;119:351-64. doi: 10.1016/s0079-6123(08)61580-0. Prog Brain Res. 1998. PMID: 10074799 Review.
-
Vasopressin acting at V1-type receptors produces membrane depolarization in neonatal rat spinal lateral column neurons.Prog Brain Res. 1998;119:275-84. doi: 10.1016/s0079-6123(08)61575-7. Prog Brain Res. 1998. PMID: 10074794 Review.
Cited by
-
Connectome of the Suprachiasmatic Nucleus: New Evidence of the Core-Shell Relationship.eNeuro. 2018 Oct 2;5(5):ENEURO.0205-18.2018. doi: 10.1523/ENEURO.0205-18.2018. eCollection 2018 Sep-Oct. eNeuro. 2018. PMID: 30283813 Free PMC article.
-
Integration of the circadian and stress systems: influence of neuropeptides and implications for alcohol consumption.J Neural Transm (Vienna). 2012 Oct;119(10):1111-20. doi: 10.1007/s00702-012-0829-4. Epub 2012 May 31. J Neural Transm (Vienna). 2012. PMID: 22648536 Review.
-
Reward-representing D1-type neurons in the medial shell of the accumbens nucleus regulate palatable food intake.Int J Obes (Lond). 2019 Apr;43(4):917-927. doi: 10.1038/s41366-018-0133-y. Epub 2018 Jun 15. Int J Obes (Lond). 2019. PMID: 29907842 Free PMC article.
-
Resynchronization Dynamics Reveal that the Ventral Entrains the Dorsal Suprachiasmatic Nucleus.J Biol Rhythms. 2017 Feb;32(1):35-47. doi: 10.1177/0748730416680904. Epub 2016 Dec 20. J Biol Rhythms. 2017. PMID: 28326909 Free PMC article.
-
Novel oxygen sensing mechanism in the spinal cord involved in cardiorespiratory responses to hypoxia.Sci Adv. 2022 Mar 25;8(12):eabm1444. doi: 10.1126/sciadv.abm1444. Epub 2022 Mar 25. Sci Adv. 2022. PMID: 35333571 Free PMC article.
References
-
- Arima H, House SB, Gainer H, Aguilera G. Neuronal activity is required for the circadian rhythm of vasopressin gene transcription in the suprachiasmatic nucleus in vitro. Endocrinology. 2002;143:4165–4171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
