Facilitation of extinction learning for contextual fear memory by PEPA: a potentiator of AMPA receptors
- PMID: 17202483
- PMCID: PMC6672292
- DOI: 10.1523/JNEUROSCI.3842-06.2007
Facilitation of extinction learning for contextual fear memory by PEPA: a potentiator of AMPA receptors
Abstract
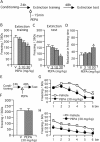
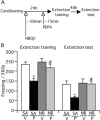
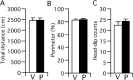
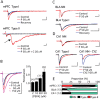
Contextual fear memory is attenuated by the re-exposure of mice to the context without aversive stimulus. This phenomenon is called extinction. Here, we report that a potentiator of AMPA receptors, 4-[2-(phenylsulfonylamino)ethylthio]-2,6-difluorophenoxyacetamide (PEPA), potently facilitates extinction learning in mice. C57BL/6J mice were exposed to novel context and stimulated by electrical footshock. After 24 h (extinction training) and 72 h (extinction test), the mice were repeatedly exposed to the context without footshock and the duration of their freezing response was measured. The duration of freezing response in the extinction test was consistently shorter than the value in extinction training. Intraperitoneal injection of PEPA 15 min before extinction training remarkably reduced the duration of freezing responses during the extinction training and test, compared with the vehicle-injected control mice. This action of PEPA on extinction was dose-dependent and inhibited by NBQX (1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide), an AMPA receptor antagonist. PEPA had no effect on acquisition and consolidation of fear memory itself. Electrophysiological studies suggested that PEPA activates the neural network much more potently in the medial prefrontal cortex (mPFC) than in the basolateral amygdala and hippocampal CA1 field. Quantitative PCR studies suggested the pronounced expression of PEPA-preferring AMPA receptor subunits (GluR3 and GluR4) and a splice variant (flop) in the mPFC. An intra-mPFC injection of PEPA facilitated the extinction much more potently than an intra-amygdala injection of PEPA did. These results suggest that PEPA facilitates extinction learning through AMPA receptor activation mainly in the mPFC.
Figures
Similar articles
-
Pharmacological discrimination of extinction and reconsolidation of contextual fear memory by a potentiator of AMPA receptors.Neuropsychopharmacology. 2009 Nov;34(12):2574-84. doi: 10.1038/npp.2009.86. Epub 2009 Aug 12. Neuropsychopharmacology. 2009. PMID: 19675539
-
Facilitating actions of an AMPA receptor potentiator upon extinction of contextually conditioned fear response in stressed mice.Neurosci Lett. 2011 Jan 25;488(3):242-6. doi: 10.1016/j.neulet.2010.11.038. Epub 2010 Nov 19. Neurosci Lett. 2011. PMID: 21094221
-
Pharmacological detection of AMPA receptor heterogeneity by use of two allosteric potentiators in rat hippocampal cultures.Br J Pharmacol. 1998 Apr;123(7):1294-303. doi: 10.1038/sj.bjp.0701707. Br J Pharmacol. 1998. PMID: 9579722 Free PMC article.
-
A novel allosteric potentiator of AMPA receptors: 4--2-(phenylsulfonylamino)ethylthio--2,6-difluoro-phenoxyaceta mide.J Neurosci. 1997 Aug 1;17(15):5760-71. doi: 10.1523/JNEUROSCI.17-15-05760.1997. J Neurosci. 1997. PMID: 9221774 Free PMC article.
-
Prefrontal mechanisms in extinction of conditioned fear.Biol Psychiatry. 2006 Aug 15;60(4):337-43. doi: 10.1016/j.biopsych.2006.03.010. Epub 2006 May 19. Biol Psychiatry. 2006. PMID: 16712801 Review.
Cited by
-
Multiple roles for orexin/hypocretin in addiction.Prog Brain Res. 2012;198:79-121. doi: 10.1016/B978-0-444-59489-1.00007-0. Prog Brain Res. 2012. PMID: 22813971 Free PMC article. Review.
-
Calcyon upregulation in adolescence impairs response inhibition and working memory in adulthood.Mol Psychiatry. 2011 Jun;16(6):672-84. doi: 10.1038/mp.2011.14. Epub 2011 Mar 15. Mol Psychiatry. 2011. PMID: 21403673 Free PMC article.
-
Stress induces insertion of calcium-permeable AMPA receptors in the OFC-BLA synapse and modulates emotional behaviours in mice.Transl Psychiatry. 2020 May 18;10(1):154. doi: 10.1038/s41398-020-0837-3. Transl Psychiatry. 2020. PMID: 32424318 Free PMC article.
-
Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons.J Neurosci. 2008 Apr 9;28(15):4028-36. doi: 10.1523/JNEUROSCI.2623-07.2008. J Neurosci. 2008. PMID: 18400902 Free PMC article.
-
Enhancing exposure-based therapy from a translational research perspective.Behav Res Ther. 2007 Sep;45(9):1987-2001. doi: 10.1016/j.brat.2007.06.006. Epub 2007 Jun 17. Behav Res Ther. 2007. PMID: 17659253 Free PMC article. Review.
References
-
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. - PubMed
-
- Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–2419. - PubMed
-
- Blanchard RJ, Blanchard DC. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. - PubMed
-
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. - PubMed
-
- Bouton ME, Kenney FA, Rosengard C. State-dependent fear extinction with two benzodiazepine tranquilizers. Behav Neurosci. 1990;104:44–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
