Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat
- PMID: 17202485
- PMCID: PMC6672294
- DOI: 10.1523/JNEUROSCI.3227-06.2007
Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat
Abstract
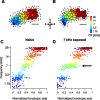
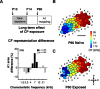
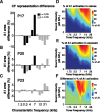
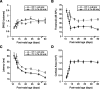
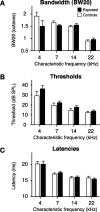
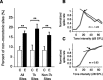
Experience-dependent plasticity during development results in the emergence of highly adapted representations of the external world in the adult brain. Previous studies have convincingly shown that the primary auditory cortex (A1) of the rat possesses a postnatal period of sensory input-driven plasticity but its precise timing (onset, duration, end) has not been defined. In the present study, we examined the effects of pure-tone exposure on the auditory cortex of developing rat pups at different postnatal ages with a high temporal resolution. We found that pure-tone exposure resulted in profound, persistent alterations in sound representations in A1 only if the exposure occurred during a brief period extending from postnatal day 11 (P11) to P13. We also found that postnatal sound exposure in this epoch led to striking alterations in the cortical representation of sound intensity.
Figures


References
-
- Astl J, Popelar J, Kvasnak E, Syka J. Comparison of response properties of neurons in the inferior colliculus of guinea pigs under different anesthetics. Audiology. 1996;35:335–345. - PubMed
-
- Bao S, Chang EF, Heiser MA, Merzenich MM. Representation of complex sounds through early experience. Soc Neurosci Abstr. 2003a;29:182.16.
-
- Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci. 2004;7:974–981. - PubMed
-
- Bartoletti A, Medini P, Berardi N, Maffei L. Environmental enrichment prevents effects of dark-rearing in the rat visual cortex. Nat Neurosci. 2004;7:215–216. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
