Potentiation of exocytosis by phospholipase C-coupled G-protein-coupled receptors requires the priming protein Munc13-1
- PMID: 17202488
- PMCID: PMC6672273
- DOI: 10.1523/JNEUROSCI.4201-06.2007
Potentiation of exocytosis by phospholipase C-coupled G-protein-coupled receptors requires the priming protein Munc13-1
Abstract
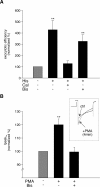
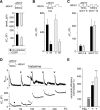
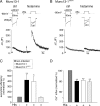
The vesicle priming protein Munc13-1 is regulated by diacylglycerol (DAG) and is therefore hypothesized to play a role in the control of neurotransmitter release by phospholipase C (PLC)-coupled receptors. We combined voltage-clamp recordings of voltage-gated Ca2+ channels (VGCCs) and high-resolution capacitance measurements to investigate the mechanism of receptor-mediated modulation of exocytosis in bovine chromaffin cells. Activation of endogenous H1 G(q)-protein-coupled receptors (G(q)PCRs) by histamine potentiated stimulus-coupled secretion despite concurrently inhibiting Ca2+ influx through VGCCs. Histamine increased the size of the readily releasable pool of vesicles and in particular a subpool of fusion-competent vesicles localized in close proximity to VGCCs. Pharmacological characterization showed that potentiation of exocytosis depended on the activation of PLC but not protein kinase C. Overexpression of wild-type Munc13-1 by adenoviral infection had no effect on histamine-induced potentiation per se, whereas DAG-insensitive Munc13-1(H567K) completely abolished it. This is the first endogenous mammalian G(q)PCR signaling pathway identified that engages Munc13-1 to increase stimulus-coupled secretion by recruiting vesicles to the immediately releasable pool. G(q)PCRs are therefore able to control exocytosis at the level of SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex formation to produce rapid, short-term potentiation of the secretory output of neurons and endocrine cells.
Figures
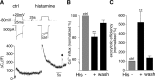

Similar articles
-
Regulation of insulin exocytosis by Munc13-1.J Biol Chem. 2003 Jul 25;278(30):27556-63. doi: 10.1074/jbc.M303203200. Epub 2003 May 13. J Biol Chem. 2003. PMID: 12871971
-
Bidirectional modulation of exocytosis by angiotensin II involves multiple G-protein-regulated transduction pathways in chromaffin cells.J Neurosci. 2000 Jul 1;20(13):4776-85. doi: 10.1523/JNEUROSCI.20-13-04776.2000. J Neurosci. 2000. PMID: 10864935 Free PMC article.
-
P/Q Ca2+ channels are functionally coupled to exocytosis of the immediately releasable pool in mouse chromaffin cells.Cell Calcium. 2008 Feb;43(2):155-64. doi: 10.1016/j.ceca.2007.04.014. Epub 2007 Jun 11. Cell Calcium. 2008. PMID: 17561253
-
Calcium signaling and exocytosis in adrenal chromaffin cells.Physiol Rev. 2006 Oct;86(4):1093-131. doi: 10.1152/physrev.00039.2005. Physiol Rev. 2006. PMID: 17015485 Review.
-
The immediately releasable vesicle pool: highly coupled secretion in chromaffin and other neuroendocrine cells.J Neurochem. 2011 Jan;116(2):155-63. doi: 10.1111/j.1471-4159.2010.07108.x. J Neurochem. 2011. PMID: 21073467 Review.
Cited by
-
Munc13 mediates klotho-inhibitable diacylglycerol-stimulated exocytotic insertion of pre-docked TRPC6 vesicles.PLoS One. 2020 Mar 5;15(3):e0229799. doi: 10.1371/journal.pone.0229799. eCollection 2020. PLoS One. 2020. PMID: 32134975 Free PMC article.
-
Lipid metabolism in the adrenal gland.Front Endocrinol (Lausanne). 2025 Jun 9;16:1577505. doi: 10.3389/fendo.2025.1577505. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40551885 Free PMC article. Review.
-
Imaging the rapid yet transient accumulation of regulatory lipids, lipid kinases, and protein kinases during membrane fusion, at sites of exocytosis of MMP-9 in MCF-7 cells.Lipids Health Dis. 2020 Aug 23;19(1):195. doi: 10.1186/s12944-020-01374-9. Lipids Health Dis. 2020. PMID: 32829709 Free PMC article.
-
Glucose-stimulated insulin secretion depends on FFA1 and Gq in neonatal mouse islets.Diabetologia. 2023 Aug;66(8):1501-1515. doi: 10.1007/s00125-023-05932-5. Epub 2023 May 23. Diabetologia. 2023. PMID: 37217659 Free PMC article.
-
Role of PI(4,5)P(2) in vesicle exocytosis and membrane fusion.Subcell Biochem. 2012;59:111-30. doi: 10.1007/978-94-007-3015-1_4. Subcell Biochem. 2012. PMID: 22374089 Free PMC article. Review.
References
-
- Ashery U, Betz A, Xu T, Brose N, Rettig J. An efficient method for infection of adrenal chromaffin cells using the Semliki Forest virus gene expression system. Eur J Cell Biol. 1999;78:525–532. - PubMed
-
- Aunis D, Langley K. Physiological aspects of exocytosis in chromaffin cells of the adrenal medulla. Acta Physiol Scand. 1999;167:89–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
