Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration
- PMID: 17203472
- PMCID: PMC2871685
- DOI: 10.1002/glia.20467
Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration
Abstract
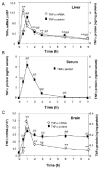
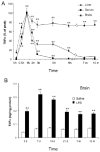
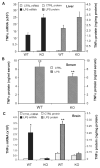
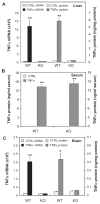
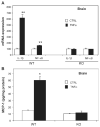
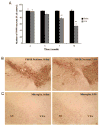
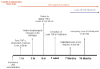
Inflammation is implicated in the progressive nature of neurodegenerative diseases, such as Parkinson's disease, but the mechanisms are poorly understood. A single systemic lipopolysaccharide (LPS, 5 mg/kg, i.p.) or tumor necrosis factor alpha (TNFalpha, 0.25 mg/kg, i.p.) injection was administered in adult wild-type mice and in mice lacking TNFalpha receptors (TNF R1/R2(-/-)) to discern the mechanisms of inflammation transfer from the periphery to the brain and the neurodegenerative consequences. Systemic LPS administration resulted in rapid brain TNFalpha increase that remained elevated for 10 months, while peripheral TNFalpha (serum and liver) had subsided by 9 h (serum) and 1 week (liver). Systemic TNFalpha and LPS administration activated microglia and increased expression of brain pro-inflammatory factors (i.e., TNFalpha, MCP-1, IL-1beta, and NF-kappaB p65) in wild-type mice, but not in TNF R1/R2(-/-) mice. Further, LPS reduced the number of tyrosine hydroxylase-immunoreactive neurons in the substantia nigra (SN) by 23% at 7-months post-treatment, which progressed to 47% at 10 months. Together, these data demonstrate that through TNFalpha, peripheral inflammation in adult animals can: (1) activate brain microglia to produce chronically elevated pro-inflammatory factors; (2) induce delayed and progressive loss of DA neurons in the SN. These findings provide valuable insight into the potential pathogenesis and self-propelling nature of Parkinson's disease.
(c) 2007 Wiley-Liss, Inc.
Figures

References
-
- Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. - PubMed
-
- Bernardini R, Kamilaris TC, Calogero AE, Johnson EO, Gomez MT, Gold PW, Chrousos GP. Interactions between tumor necrosis factor-a, hypothalamic corticotropin-releasing hormone, and adrenocorticotropin secretion in the rat. Endocrinology. 1990;126:2876–2881. - PubMed
-
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. - PubMed
-
- Block ML, Wu X, Pei Z, Li G, Wang T, Qin L, Wilson B, Yang J, Hong JS, Veronesi B. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: The role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004;18:1618–1620. - PubMed
-
- Carvey PM, Chang Q, Lipton JW, Ling Z. Prenatal exposure to the bacteriotoxin lipopolysaccharide leads to long-term losses of dopamine neurons in offspring: A potential, new model of Parkinson's disease. Front Biosci. 2003;8:S826–S837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

