Contribution of altered signal transduction associated to glutamate receptors in brain to the neurological alterations of hepatic encephalopathy
- PMID: 17203513
- PMCID: PMC4087535
- DOI: 10.3748/wjg.v12.i48.7737
Contribution of altered signal transduction associated to glutamate receptors in brain to the neurological alterations of hepatic encephalopathy
Abstract
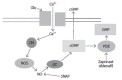
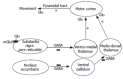
Patients with liver disease may present hepatic encephalopathy (HE), a complex neuropsychiatric syndrome covering a wide range of neurological alterations, including cognitive and motor disturbances. HE reduces the quality of life of the patients and is associated with poor prognosis. In the worse cases HE may lead to coma or death. The mechanisms leading to HE which are not well known are being studied using animal models. The neurological alterations in HE are a consequence of impaired cerebral function mainly due to alterations in neurotransmission. We review here some studies indicating that alterations in neurotransmission associated to different types of glutamate receptors are responsible for some of the cognitive and motor alterations present in HE. These studies show that the function of the signal transduction pathway glutamate-nitric oxide-cGMP associated to the NMDA type of glutamate receptors is impaired in brain in vivo in HE animal models as well as in brain of patients died of HE. Activation of NMDA receptors in brain activates this pathway and increases cGMP. In animal models of HE this increase in cGMP induced by activation of NMDA receptors is reduced, which is responsible for the impairment in learning ability in these animal models. Increasing cGMP by pharmacological means restores learning ability in rats with HE and may be a new therapeutic approach to improve cognitive function in patients with HE. However, it is necessary to previously assess the possible secondary effects. Patients with HE may present psychomotor slowing, hypokinesia and bradykinesia. Animal models of HE also show hypolocomotion. It has been shown in rats with HE that hypolocomotion is due to excessive activation of metabotropic glutamate receptors (mGluRs) in substantia nigra pars reticulata. Blocking mGluR1 in this brain area normalizes motor activity in the rats, suggesting that a similar treatment for patients with HE could be useful to treat psychomotor slowing and hypokinesia. However, the possible secondary effects of mGluR1 antagonists should be previously evaluated. These studies are setting the basis for designing therapeutic procedures to specifically treat the individual neurological alterations in patients with HE.
Figures
References
-
- Felipo V, Butterworth RF. Neurobiology of ammonia. Prog Neurobiol. 2002;67:259–279. - PubMed
-
- Shawcross DL, Davies NA, Williams R, Jalan R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol. 2004;40:247–254. - PubMed
-
- Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V, Rodés J. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30:890–895. - PubMed
-
- Hui AY, Chan HL, Leung NW, Hung LC, Chan FK, Sung JJ. Survival and prognostic indicators in patients with hepatitis B virus-related cirrhosis after onset of hepatic decompensation. J Clin Gastroenterol. 2002;34:569–572. - PubMed
-
- Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis. 2004;19:253–267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

