Chicken ovalbumin upstream promoter transcription factor II regulates uncoupling protein 3 gene transcription in Phodopus sungorus
- PMID: 17204145
- PMCID: PMC1779797
- DOI: 10.1186/1471-2199-8-1
Chicken ovalbumin upstream promoter transcription factor II regulates uncoupling protein 3 gene transcription in Phodopus sungorus
Abstract
Background: Ucp3 is an integral protein of the inner mitochondrial membrane with a role in lipid metabolism preventing deleterious effects of fatty acids in states of high lipid oxidation. Ucp3 is expressed in brown adipose tissue and skeletal muscle and controlled by a transcription factor complex including PPARalpha, MyoD and the histone acetyltransferase p300. Several studies have demonstrated interaction of these factors with chicken ovalbumin upstream promoter transcription factor II (Coup-TFII). This nuclear receptor is involved in organogenesis and other developmental processes including skeletal muscle development, but also co-regulates a number of metabolic genes. In this study we in silico analyzed the upstream region of Ucp3 of the Djungarian hamster Phodopus sungorus and identified several putative response elements for Coup-TFII. We therefore investigated whether Coup-TFII is a further player in the transcriptional control of the Ucp3 gene in rodents.
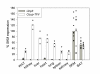
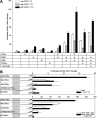
Results: By quantitative PCR we demonstrated a positive correlation of Coup-TFII and Ucp3 mRNA expression in skeletal muscle and brown adipose tissue in response to food deprivation and cold exposure, respectively. In reporter gene assays Coup-TFII enhanced transactivation of the Ucp3 promoter conveyed by MyoD, PPARalpha, RXRalpha and/or p300. Using deletions and mutated constructs, we identified a Coup-TFII enhancer element 816-840 bp upstream of the transcriptional start site. Binding of Coup-TFII to this upstream enhancer was confirmed in electrophoretic mobility shift and supershift assays.
Conclusion: Transcriptional regulation of the Coup-TFII gene in response to starvation and cold exposure seems to be the regulatory mechanism of Ucp3 mRNA expression in brown adipose and skeletal muscle tissue determining the final appropriate rate of transcript synthesis. These findings add a crucial component to the complex transcriptional machinery controlling expression of Ucp3. Given the substantial evidence for a function of Ucp3 in lipid metabolism, Coup-TFII may not only be a negative regulator of glucose responsive genes but also transactivate genes involved in lipid metabolism.
Figures

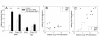



Similar articles
-
Chicken ovalbumin upstream promoter-transcription factor II regulates nuclear receptor, myogenic, and metabolic gene expression in skeletal muscle cells.Physiol Genomics. 2011 Feb 24;43(4):213-27. doi: 10.1152/physiolgenomics.00195.2010. Epub 2010 Nov 30. Physiol Genomics. 2011. PMID: 21119012
-
The INSL3 gene is a direct target for the orphan nuclear receptor, COUP-TFII, in Leydig cells.J Mol Endocrinol. 2014 Aug;53(1):43-55. doi: 10.1530/JME-13-0290. Epub 2014 Apr 29. J Mol Endocrinol. 2014. PMID: 24780841
-
Chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) and hepatocyte nuclear factor 4gamma (HNF-4gamma) and HNF-4alpha regulate the bovine growth hormone receptor 1A promoter through a common DNA element.J Mol Endocrinol. 2004 Jun;32(3):947-61. doi: 10.1677/jme.0.0320947. J Mol Endocrinol. 2004. PMID: 15171724
-
COUP-TFII revisited: Its role in metabolic gene regulation.Steroids. 2019 Jan;141:63-69. doi: 10.1016/j.steroids.2018.11.013. Epub 2018 Nov 24. Steroids. 2019. PMID: 30481528 Free PMC article. Review.
-
Interaction of the glucocorticoid receptor and the chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII): implications for the actions of glucocorticoids on glucose, lipoprotein, and xenobiotic metabolism.Ann N Y Acad Sci. 2004 Jun;1024:72-85. doi: 10.1196/annals.1321.006. Ann N Y Acad Sci. 2004. PMID: 15265774 Review.
Cited by
-
A novel SP1/SP3 dependent intronic enhancer governing transcription of the UCP3 gene in brown adipocytes.PLoS One. 2013 Dec 31;8(12):e83426. doi: 10.1371/journal.pone.0083426. eCollection 2013. PLoS One. 2013. PMID: 24391766 Free PMC article.
-
Decoding the aroma of Rosa canina L.: Chemical composition and gene expression.PLoS One. 2025 Jan 24;20(1):e0316324. doi: 10.1371/journal.pone.0316324. eCollection 2025. PLoS One. 2025. PMID: 39854304 Free PMC article.
-
Identification of an appropriate reference gene for normalization of qRT-PCR expression analyses in human breast cancer cell lines: application to L-arginine depletion studies.J Cancer Res Clin Oncol. 2025 Mar 26;151(3):122. doi: 10.1007/s00432-025-06165-2. J Cancer Res Clin Oncol. 2025. PMID: 40133575 Free PMC article.
-
In silico investigation of uncoupling protein function in avian genomes.Front Vet Sci. 2023 Jan 19;9:1085112. doi: 10.3389/fvets.2022.1085112. eCollection 2022. Front Vet Sci. 2023. PMID: 36744229 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

