Mucus secretion by single tracheal submucosal glands from normal and cystic fibrosis transmembrane conductance regulator knockout mice
- PMID: 17204498
- PMCID: PMC2075436
- DOI: 10.1113/jphysiol.2006.123653
Mucus secretion by single tracheal submucosal glands from normal and cystic fibrosis transmembrane conductance regulator knockout mice
Abstract
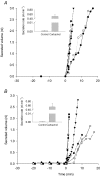
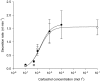
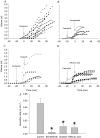
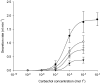
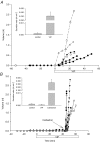
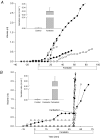
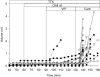
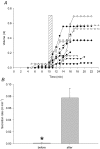
Submucosal glands line the cartilaginous airways and produce most of the antimicrobial mucus that keeps the airways sterile. The glands are defective in cystic fibrosis (CF), but how this impacts airway health remains uncertain. Although most CF mouse strains exhibit mild airway defects, those with the C57Bl/6 genetic background have increased airway pathology and susceptibility to Pseudomonas. Thus, they offer the possibility of studying whether, and if so how, abnormal submucosal gland function contributes to CF airway disease. We used optical methods to study fluid secretion by individual glands in tracheas from normal, wild-type (WT) mice and from cystic fibrosis transmembrane conductance regulator (CFTR) knockout mice (Cftr(m1UNC)/Cftr(m1UNC); CF mice). Glands from WT mice qualitatively resembled those in humans by responding to carbachol and vasoactive intestinal peptide (VIP), although the relative rates of VIP- and forskolin-stimulated secretion were much lower in mice than in large mammals. The pharmacology of mouse gland secretion was also similar to that in humans; adding bumetanide or replacement of HCO(3)(-) by Hepes reduced the carbachol response by approximately 50%, and this inhibition increased to 80% when both manoeuvres were performed simultaneously. It is important to note that glands from CFTR knockout mice responded to carbachol but did not secrete when exposed to VIP or forskolin, as has been shown previously for glands from CF patients. Tracheal glands from WT and CF mice both had robust secretory responses to electrical field stimulation that were blocked by tetrodotoxin. It is interesting that local irritation of the mucosa using chili pepper oil elicited secretion from WT glands but did not stimulate glands from CF mice. These results clarify the mechanisms of murine submucosal gland secretion and reveal a novel defect in local regulation of glands lacking CFTR which may also compromise airway defence in CF patients.
Figures


Comment in
-
Cystic fibrosis mice rehabilitated for studies of airway gland dysfunction.J Physiol. 2007 Apr 1;580(Pt 1):7-8. doi: 10.1113/jphysiol.2007.129361. Epub 2007 Feb 22. J Physiol. 2007. PMID: 17317740 Free PMC article. No abstract available.
Similar articles
-
Synergistic airway gland mucus secretion in response to vasoactive intestinal peptide and carbachol is lost in cystic fibrosis.J Clin Invest. 2007 Oct;117(10):3118-27. doi: 10.1172/JCI31992. J Clin Invest. 2007. PMID: 17853942 Free PMC article.
-
HCO3- transport in relation to mucus secretion from submucosal glands.JOP. 2001 Jul;2(4 Suppl):280-4. JOP. 2001. PMID: 11875272 Review.
-
Substance P stimulates human airway submucosal gland secretion mainly via a CFTR-dependent process.J Clin Invest. 2009 May;119(5):1189-200. doi: 10.1172/JCI37284. Epub 2009 Apr 20. J Clin Invest. 2009. PMID: 19381016 Free PMC article.
-
Hyposecretion of fluid from tracheal submucosal glands of CFTR-deficient pigs.J Clin Invest. 2010 Sep;120(9):3161-6. doi: 10.1172/JCI43466. Epub 2010 Aug 25. J Clin Invest. 2010. PMID: 20739758 Free PMC article.
-
Parasympathetic control of airway submucosal glands: central reflexes and the airway intrinsic nervous system.Auton Neurosci. 2007 Apr 30;133(1):35-54. doi: 10.1016/j.autneu.2007.01.008. Epub 2007 Mar 9. Auton Neurosci. 2007. PMID: 17350348 Free PMC article. Review.
Cited by
-
Substance P stimulates CFTR-dependent fluid secretion by mouse tracheal submucosal glands.Pflugers Arch. 2008 Nov;457(2):529-37. doi: 10.1007/s00424-008-0527-0. Epub 2008 May 29. Pflugers Arch. 2008. PMID: 18509672
-
Fluid secretion by submucosal glands of the tracheobronchial airways.Respir Physiol Neurobiol. 2007 Dec 15;159(3):271-7. doi: 10.1016/j.resp.2007.06.017. Epub 2007 Jul 7. Respir Physiol Neurobiol. 2007. PMID: 17707699 Free PMC article. Review.
-
Synergistic airway gland mucus secretion in response to vasoactive intestinal peptide and carbachol is lost in cystic fibrosis.J Clin Invest. 2007 Oct;117(10):3118-27. doi: 10.1172/JCI31992. J Clin Invest. 2007. PMID: 17853942 Free PMC article.
-
Defective fluid secretion from submucosal glands of nasal turbinates from CFTR-/- and CFTR (ΔF508/ΔF508) pigs.PLoS One. 2011;6(8):e24424. doi: 10.1371/journal.pone.0024424. Epub 2011 Aug 31. PLoS One. 2011. PMID: 21935358 Free PMC article.
-
Correlation of apical fluid-regulating channel proteins with lung function in human COPD lungs.PLoS One. 2014 Oct 17;9(10):e109725. doi: 10.1371/journal.pone.0109725. eCollection 2014. PLoS One. 2014. PMID: 25329998 Free PMC article.
References
-
- Ballard ST, Trout L, Bebok Z, Sorscher EJ, Crews A. CFTR involvement in chloride, bicarbonate, and liquid secretion by airway submucosal glands. Am J Physiol Lung Cell Mol Physiol. 1999;277:L694–L699. - PubMed
-
- Ballard ST, Trout L, Garrison J, Inglis SK. Ionic mechanism of forskolin-induced liquid secretion by porcine bronchi. Am J Physiol Lung Cell Mol Physiol. 2006;290:L97–L104. - PubMed
-
- Barnes PJ. Neurogenic inflammation in the airways. Respir Physiol. 2001;125:145–154. - PubMed
-
- Borthwick DW, West JD, Keighren MA, Flockhart JH, Innes BA, Dorin JR. Murine submucosal glands are clonally derived and show a cystic fibrosis gene-dependent distribution pattern. Am J Respir Cell Mol Biol. 1999;20:1181–1189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

