Permeating protons contribute to tachyphylaxis of the acid-sensing ion channel (ASIC) 1a
- PMID: 17204502
- PMCID: PMC2151377
- DOI: 10.1113/jphysiol.2006.120733
Permeating protons contribute to tachyphylaxis of the acid-sensing ion channel (ASIC) 1a
Abstract
The homomeric acid-sensing ion channel 1a (ASIC1a) is a H+-activated ion channel with important physiological functions and pathophysiological impact in the central nervous system. Here we show that homomeric ASIC1a is distinguished from other ASICs by a reduced response to successive acid stimulations. Such a reduced response is called tachyphylaxis. We show that tachyphylaxis depends on H+ permeating through ASIC1a, that tachyphylaxis is attenuated by extracellular Ca2+, and that tachyphylaxis is probably linked to Ca2+ permeability of ASIC1a. Moreover, we provide evidence that tachyphylaxis is probably due to a long-lived inactive state of ASIC1a. A deeper understanding of ASIC1a tachyphylaxis may lead to pharmacological control of ASIC1a activity that could be of potential benefit for the treatment of stroke.
Figures
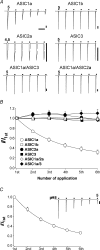
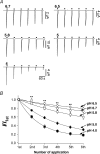

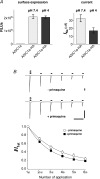

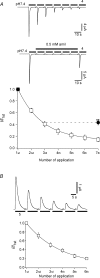



Similar articles
-
Identification of the Ca2+ blocking site of acid-sensing ion channel (ASIC) 1: implications for channel gating.J Gen Physiol. 2004 Oct;124(4):383-94. doi: 10.1085/jgp.200308973. J Gen Physiol. 2004. PMID: 15452199 Free PMC article.
-
The tarantula toxin psalmotoxin 1 inhibits acid-sensing ion channel (ASIC) 1a by increasing its apparent H+ affinity.J Gen Physiol. 2005 Jul;126(1):71-9. doi: 10.1085/jgp.200509303. Epub 2005 Jun 13. J Gen Physiol. 2005. PMID: 15955877 Free PMC article.
-
Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent.J Gen Physiol. 2006 Mar;127(3):267-76. doi: 10.1085/jgp.200509409. Epub 2006 Feb 14. J Gen Physiol. 2006. PMID: 16505147 Free PMC article.
-
Acid-sensing ion channels: advances, questions and therapeutic opportunities.Trends Neurosci. 2006 Oct;29(10):578-86. doi: 10.1016/j.tins.2006.06.014. Epub 2006 Aug 7. Trends Neurosci. 2006. PMID: 16891000 Review.
-
Peptides inhibitors of acid-sensing ion channels.Toxicon. 2007 Feb;49(2):271-84. doi: 10.1016/j.toxicon.2006.09.026. Epub 2006 Oct 4. Toxicon. 2007. PMID: 17113616 Review.
Cited by
-
Molecular determinants of proton selectivity and gating in the red-light activated channelrhodopsin Chrimson.Sci Rep. 2017 Aug 30;7(1):9928. doi: 10.1038/s41598-017-09600-8. Sci Rep. 2017. PMID: 28855540 Free PMC article.
-
Molecular mechanism and structural basis of small-molecule modulation of the gating of acid-sensing ion channel 1.Commun Biol. 2021 Feb 9;4(1):174. doi: 10.1038/s42003-021-01678-1. Commun Biol. 2021. PMID: 33564124 Free PMC article.
-
Dynamic conformational changes of acid-sensing ion channels in different desensitizing conditions.Biophys J. 2024 Jul 16;123(14):2122-2135. doi: 10.1016/j.bpj.2024.03.038. Epub 2024 Mar 28. Biophys J. 2024. PMID: 38549370 Free PMC article.
-
Acid-sensing ion channels in neurones of the rat suprachiasmatic nucleus.J Physiol. 2009 Apr 15;587(Pt 8):1727-37. doi: 10.1113/jphysiol.2008.166918. Epub 2009 Mar 2. J Physiol. 2009. PMID: 19255120 Free PMC article.
-
Atomic level characterization of the nonproton ligand-sensing domain of ASIC3 channels.J Biol Chem. 2011 Jul 15;286(28):24996-5006. doi: 10.1074/jbc.M111.239558. Epub 2011 May 17. J Biol Chem. 2011. PMID: 21586569 Free PMC article.
References
-
- Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem. 2004;279:18296–18305. - PubMed
-
- Babini E, Paukert M, Geisler HS, Gründer S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1) J Biol Chem. 2002;277:41597–41603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

