Effect of chondroitin sulphate in symptomatic knee osteoarthritis: a multicentre, randomised, double-blind, placebo-controlled study
- PMID: 17204566
- PMCID: PMC1954603
- DOI: 10.1136/ard.2006.059899
Effect of chondroitin sulphate in symptomatic knee osteoarthritis: a multicentre, randomised, double-blind, placebo-controlled study
Abstract
Objective: To evaluate the efficacy and tolerability of chondroitin sulphate (chondroitin sulphate) in knee osteoarthritis.
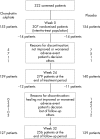
Patients and methods: A 24-week, randomised placebo-controlled trial of chondroitin sulphate (1 g/day) in patients with symptomatic knee osteoarthritis as measured on a visual analogue scale. Pain on daily activities and Lequesne's Index were the primary efficacy criteria. Secondary outcomes included the rate of responders according to the outcome measures in rheumatoid arthritis clinical trials of the Osteoarthritis Research Society International (OMERACT-OARSI) criteria, quality of life, patient's/physician's global assessments and carry-over effect after treatment. Biochemical markers of bone (CTX-I), cartilage (CTX-II) and synovium (hyaluronic acid) metabolism were also measured. Safety was assessed by recording adverse events (AEs). Statistical analysis was performed on the inter-group differences in the intention-to-treat population.
Results: 307 patients were included in the study. 28 (9%) patients discontinued the study because of lack of efficacy or AEs. At the end of treatment, the decrease in pain was -26.2 (24.9) and -19.9 (23.5) mm and improved function was -2.4 (3.4) (-25%) and -1.7 (3.3) (-17%) in the chondroitin sulphate and placebo groups, respectively (p = 0.029 and 0.109). The OMERACT-OARSI responder rate was 68% in the chondroitin sulphate and 56% in the placebo group (p = 0.03). The investigator's assessments and short form 12 (SF-12) physical component reported improvement more frequently in the chondroitin sulphate than in the placebo group (p = 0.044 and 0.021, respectively). No significant difference was observed between treatment groups for changes in biomarkers over 24 weeks. However, there was a significant difference between non-responders and responders according to the OARSI criteria for 24-week changes of CTX-I (p = 0.018) and CTX-II (p = 0.014). Tolerance was considered to be satisfactory.
Conclusion: This study failed to show an efficacy of chondroitin sulphate on the two primary criteria considered together, although chondroitin sulphate was slightly more effective than placebo on pain, OMERACT-OARSI response rate, investigator's assessment and quality of life.
Conflict of interest statement
Competing interests: BM was reimbursed by the Pierre Fabre Company for attending the Boston OARSI meeting where the trial was first presented as a poster. MZ and MH are employees of Pierre Fabre. PG was funded to perform the biochemical analyses.
Similar articles
-
Combined Treatment With Chondroitin Sulfate and Glucosamine Sulfate Shows No Superiority Over Placebo for Reduction of Joint Pain and Functional Impairment in Patients With Knee Osteoarthritis: A Six-Month Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial.Arthritis Rheumatol. 2017 Jan;69(1):77-85. doi: 10.1002/art.39819. Arthritis Rheumatol. 2017. PMID: 27477804 Clinical Trial.
-
Effect of chondroitin sulfate on soluble biomarkers of osteoarthritis: a method to analyze and interpret the results from an open-label trial in unilateral knee osteoarthritis patients.BMC Musculoskelet Disord. 2016 Oct 6;17(1):416. doi: 10.1186/s12891-016-1268-4. BMC Musculoskelet Disord. 2016. PMID: 27716158 Free PMC article. Clinical Trial.
-
Effectiveness of chondroitin sulphate in patients with concomitant knee osteoarthritis and psoriasis: a randomized, double-blind, placebo-controlled study.Osteoarthritis Cartilage. 2010 Jun;18 Suppl 1:S32-40. doi: 10.1016/j.joca.2010.01.018. Epub 2010 May 10. Osteoarthritis Cartilage. 2010. PMID: 20399899 Clinical Trial.
-
Nutraceuticals as therapeutic agents in osteoarthritis. The role of glucosamine, chondroitin sulfate, and collagen hydrolysate.Rheum Dis Clin North Am. 1999 May;25(2):379-95. doi: 10.1016/s0889-857x(05)70074-0. Rheum Dis Clin North Am. 1999. PMID: 10356424 Review.
-
Chondroitin sulphate for symptomatic osteoarthritis: critical appraisal of meta-analyses.Curr Med Res Opin. 2008 May;24(5):1303-8. doi: 10.1185/030079908x297231. Epub 2008 Apr 15. Curr Med Res Opin. 2008. PMID: 18416884 Review.
Cited by
-
Evaluation of the effect of salmon nasal proteoglycan on biomarkers for cartilage metabolism in individuals with knee joint discomfort: A randomized double-blind placebo-controlled clinical study.Exp Ther Med. 2017 Jul;14(1):115-126. doi: 10.3892/etm.2017.4454. Epub 2017 May 11. Exp Ther Med. 2017. PMID: 28672901 Free PMC article.
-
Kinetics and Retention of Polystyrenesulfonate for Proteoglycan Replacement in Cartilage.Biomacromolecules. 2024 Sep 9;25(9):5819-5833. doi: 10.1021/acs.biomac.4c00479. Epub 2024 Aug 14. Biomacromolecules. 2024. PMID: 39142342 Free PMC article.
-
Nutraceuticals in the management of osteoarthritis : a critical review.Drugs Aging. 2012 Sep;29(9):717-31. doi: 10.1007/s40266-012-0006-3. Drugs Aging. 2012. PMID: 23018608 Review.
-
Improvement in Impaired Social Cognition but Not Seizures by Everolimus in a Child with Tuberous Sclerosis-Associated Autism through Increased Serum Antioxidant Proteins and Oxidant/Antioxidant Status.Case Rep Pediatr. 2019 Nov 23;2019:2070619. doi: 10.1155/2019/2070619. eCollection 2019. Case Rep Pediatr. 2019. PMID: 31871809 Free PMC article.
-
Symptom-modifying effect of chondroitin sulfate in knee osteoarthritis: a meta-analysis of randomized placebo-controlled trials performed with structum(®).Open Rheumatol J. 2012;6:183-9. doi: 10.2174/1874312901206010183. Epub 2012 Jul 25. Open Rheumatol J. 2012. PMID: 22870166 Free PMC article.
References
-
- Jordan K M, Arden N K, Doherty M, Bannwarth B, Bijlsma J W J, Dieppe P.et al EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003621145–1155. - PMC - PubMed
-
- American College of Rheumatology Subcommittee on Osteoarthritis guidelines Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum 2000431905–1915. - PubMed
-
- Avouac B, Dropsy R. Méthodologie des essais thérapeutiques des médicaments de base de l'arthrose. Thérapie 199348315–319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources