Vasopressin-induced vasoconstriction: two concentration-dependent signaling pathways
- PMID: 17204577
- PMCID: PMC2580829
- DOI: 10.1152/japplphysiol.00825.2006
Vasopressin-induced vasoconstriction: two concentration-dependent signaling pathways
Abstract
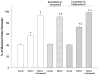
Current scientific literature generally attributes the vasoconstrictor effects of [Arg(8)]vasopressin (AVP) to the activation of phospholipase C (PLC) and consequent release of Ca(2+) from the sarcoplasmic reticulum. However, half-maximal activation of PLC requires nanomolar concentrations of AVP, whereas vasoconstriction occurs when circulating concentrations of AVP are orders of magnitude lower. Using cultured vascular smooth muscle cells, we previously identified a novel Ca(2+) signaling pathway activated by 10-100 pM AVP. This pathway is distinguished from the PLC pathway by its dependence on protein kinase C (PKC) and L-type voltage-sensitive Ca(2+) channels (VSCC). In the present study, we used isolated, pressurized rat mesenteric arteries to examine the contributions of these different Ca(2+) signaling mechanisms to AVP-induced vasoconstriction. AVP (10(-14)-10(-6) M) induced a concentration-dependent constriction of arteries that was reversible with a V(1a) vasopressin receptor antagonist. Half-maximal vasoconstriction at 30 pM AVP was prevented by blockade of VSCC with verapamil (10 microM) or by PKC inhibition with calphostin-C (250 nM) or Ro-31-8220 (1 microM). In contrast, acute vasoconstriction induced by 10 nM AVP (maximal) was insensitive to blockade of VSCC or PKC inhibition. However, after 30 min, the remaining vasoconstriction induced by 10 nM AVP was partially dependent on PKC activation and almost fully dependent on VSCC. These results suggest that different Ca(2+) signaling mechanisms contribute to AVP-induced vasoconstriction over different ranges of AVP concentration. Vasoconstrictor actions of AVP, at concentrations of AVP found within the systemic circulation, utilize a Ca(2+) signaling pathway that is dependent on PKC activation and can be inhibited by Ca(2+) channel blockers.
Figures






Similar articles
-
Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance.J Pharmacol Exp Ther. 2008 May;325(2):475-83. doi: 10.1124/jpet.107.135764. Epub 2008 Feb 13. J Pharmacol Exp Ther. 2008. PMID: 18272810 Free PMC article.
-
Ca2+ signalling in rat vascular smooth muscle cells: a role for protein kinase C at physiological vasoconstrictor concentrations of vasopressin.J Physiol. 2000 May 1;524 Pt 3(Pt 3):821-31. doi: 10.1111/j.1469-7793.2000.00821.x. J Physiol. 2000. PMID: 10790161 Free PMC article.
-
Signaling mechanisms for the selective vasoconstrictor effect of norbormide on the rat small arteries.J Pharmacol Exp Ther. 2001 Feb;296(2):458-63. J Pharmacol Exp Ther. 2001. PMID: 11160631
-
The cellular mechanisms underlying the inhibitory effects of isoflurane and sevoflurane on arginine vasopressin-induced vasoconstriction.J Anesth. 2010 Dec;24(6):893-900. doi: 10.1007/s00540-010-1033-z. Epub 2010 Oct 17. J Anesth. 2010. PMID: 20953965
-
Endothelin-1 and vasopressin signalling in blood vessels of young SHR in comparison to adult SHR.Hypertens Res. 1996 Jun;19(2):121-32. doi: 10.1291/hypres.19.121. Hypertens Res. 1996. PMID: 10968205
Cited by
-
High salt intake increases blood pressure via BDNF-mediated downregulation of KCC2 and impaired baroreflex inhibition of vasopressin neurons.Neuron. 2015 Feb 4;85(3):549-60. doi: 10.1016/j.neuron.2014.12.048. Epub 2015 Jan 22. Neuron. 2015. PMID: 25619659 Free PMC article.
-
BDNF downregulates β-adrenergic receptor-mediated hypotensive mechanisms in the paraventricular nucleus of the hypothalamus.Am J Physiol Heart Circ Physiol. 2019 Dec 1;317(6):H1258-H1271. doi: 10.1152/ajpheart.00478.2019. Epub 2019 Oct 11. Am J Physiol Heart Circ Physiol. 2019. PMID: 31603352 Free PMC article.
-
Transcriptomic identification of starfish neuropeptide precursors yields new insights into neuropeptide evolution.Open Biol. 2016 Feb;6(2):150224. doi: 10.1098/rsob.150224. Open Biol. 2016. PMID: 26865025 Free PMC article.
-
Effect of Arginine Vasopressin on Intraoperative Hypotension Caused by Oral Administration of 5-Aminolevulinic Acid.Case Rep Anesthesiol. 2023 May 6;2023:1745373. doi: 10.1155/2023/1745373. eCollection 2023. Case Rep Anesthesiol. 2023. PMID: 37192960 Free PMC article.
-
Investigation of possible underlying mechanisms behind water-induced glucose reduction in adults with high copeptin.Sci Rep. 2021 Dec 29;11(1):24481. doi: 10.1038/s41598-021-04224-5. Sci Rep. 2021. PMID: 34966186 Free PMC article. Clinical Trial.
References
-
- Ahn SC, Kim SJ, So I, Kim KW. Inhibitory effect of phorbol 12,13 dibutyrate on carbachol-activated nonselective cationic current in guineapig gastric myocytes. Pflügers Arch. 1997;434:505–507. - PubMed
-
- Altura BM. Dose-response relationships for arginine vasopressin and synthetic analogs on three types of rat blood vessels: possible evidence for regional differences in vasopressin receptor sites within a mammal. J Pharmacol Exp Ther. 1975;193:413–423. - PubMed
-
- Ang VT, Jenkins JS. Neurohypophysial hormones in the adrenal medulla. J Clin Endocrinol Metab. 1984;58:688–691. - PubMed
-
- Baylis PH, Robertson GL. Osmotic and non-osmotic stimulation of vasopressin release [proceedings] J Endocrinol. 1979;83:39P–40P. - PubMed
-
- Bonev AD, Jaggar JH, Rubart M, Nelson MT. Activators of protein kinase C decrease Ca2+ spark frequency in smooth muscle cells from cerebral arteries. Am J Physiol Cell Physiol. 1997;273:C2090–C2095. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

