Role of TAB1 in nitric oxide-induced p38 activation in insulin-producing cells
- PMID: 17205106
- PMCID: PMC1752226
- DOI: 10.7150/ijbs.3.71
Role of TAB1 in nitric oxide-induced p38 activation in insulin-producing cells
Abstract
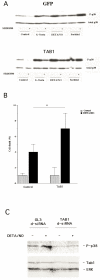
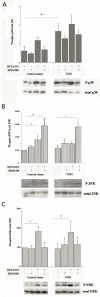
The aim of present study was to elucidate the role of TAB1 in nitric oxide-induced activation of p38 MAPK. For this purpose we over-expressed TAB1 in insulin-producing beta-TC6 cells. We observed in cells transiently over-expressing TAB1 that p38 activation was enhanced in response to DETA/NONOate. A lowering of TAB1 levels, using the siRNA technique, resulted in the opposite effect. The DETA/NONOate-induced cell death rate was increased in cells transiently overexpressing TAB1. In stable beta-TC6 cell clones with very high TAB1 levels p38 phosphorylation was enhanced also at basal conditions. DETA/NONOate increased also the phosphorylation of JNK and ERK in beta-TC6 cells, but these events were not affected by TAB1. Interestingly, the inhibitory effect of SB203580 on p38 phosphorylation was paralleled by a stimulatory effect on JNK phosphorylation and an inhibitory effect on ERK phosphorylation. In summary, we propose that TAB1 promotes nitric oxide-induced p38 autophosphorylation. In addition, nitric oxide-induced p38 activation seems to promote JNK inhibition and ERK activation, but this effect appears to not require TAB1. A better understanding of how the TAB1/p38 pathway promotes beta-cell death in response to nitric oxide might help in the development of novel pharmacological approaches in the treatment of diabetes.
Conflict of interest statement
Conflict of Interests: The authors have declared that no conflict of interest exists.
Figures

Similar articles
-
Transforming growth factor-beta-activated protein kinase 1-binding protein (TAB)-1alpha, but not TAB1beta, mediates cytokine-induced p38 mitogen-activated protein kinase phosphorylation and cell death in insulin-producing cells.Endocrinology. 2008 Jan;149(1):302-9. doi: 10.1210/en.2007-0690. Epub 2007 Oct 11. Endocrinology. 2008. PMID: 17932218
-
Role of MKK3 and p38 MAPK in cytokine-induced death of insulin-producing cells.Biochem J. 2006 Jan 1;393(Pt 1):129-39. doi: 10.1042/BJ20050814. Biochem J. 2006. PMID: 16097952 Free PMC article.
-
G protein-coupled receptors activate p38 MAPK via a non-canonical TAB1-TAB2- and TAB1-TAB3-dependent pathway in endothelial cells.J Biol Chem. 2019 Apr 12;294(15):5867-5878. doi: 10.1074/jbc.RA119.007495. Epub 2019 Feb 13. J Biol Chem. 2019. PMID: 30760523 Free PMC article.
-
Low concentrations of nitric oxide (NO) induced cell death in PC12 cells through activation of p38 mitogen-activated protein kinase (p38 MAPK) but not via extracellular signal-regulated kinases (ERK1/2) or c-Jun N-terminal protein kinase (JNK).Neurosci Lett. 2006 Jan 16;392(3):170-3. doi: 10.1016/j.neulet.2005.09.012. Epub 2005 Sep 28. Neurosci Lett. 2006. PMID: 16198052
-
Protein Phosphorylation in Cancer: Role of Nitric Oxide Signaling Pathway.Biomolecules. 2021 Jul 10;11(7):1009. doi: 10.3390/biom11071009. Biomolecules. 2021. PMID: 34356634 Free PMC article. Review.
Cited by
-
Short-Term Protocols to Obtain Insulin-Producing Cells from Rat Adipose Tissue: Signaling Pathways and In Vivo Effect.Int J Mol Sci. 2019 May 18;20(10):2458. doi: 10.3390/ijms20102458. Int J Mol Sci. 2019. PMID: 31109026 Free PMC article.
-
Roles and Interaction of the MAPK Signaling Cascade in Aβ25-35-Induced Neurotoxicity Using an Isolated Primary Hippocampal Cell Culture System.Cell Mol Neurobiol. 2021 Oct;41(7):1497-1507. doi: 10.1007/s10571-020-00912-4. Epub 2020 Jun 29. Cell Mol Neurobiol. 2021. PMID: 32601776 Free PMC article.
-
Is NO the Answer? The Nitric Oxide Pathway Can Support Bone Morphogenetic Protein 2 Mediated Signaling.Cells. 2019 Oct 18;8(10):1273. doi: 10.3390/cells8101273. Cells. 2019. PMID: 31635347 Free PMC article.
-
Protein kinases: mechanisms and downstream targets in inflammation-mediated obesity and insulin resistance.Mol Cell Biochem. 2017 Feb;426(1-2):27-45. doi: 10.1007/s11010-016-2878-8. Epub 2016 Nov 21. Mol Cell Biochem. 2017. PMID: 27868170 Free PMC article. Review.
-
RIP1 potentiates BPDE-induced transformation in human bronchial epithelial cells through catalase-mediated suppression of excessive reactive oxygen species.Carcinogenesis. 2013 Sep;34(9):2119-28. doi: 10.1093/carcin/bgt143. Epub 2013 Apr 30. Carcinogenesis. 2013. PMID: 23633517 Free PMC article.
References
-
- Eizirik DL, Pavlovic D. Is there role for NO in β-cell dysfunction and damage in IDDM? Diabetes/metab Rev. 1997;13:293–307. - PubMed
-
- Welsh N, Eizirik DL, Bendtzen K et al. Interleukin-1β-induced NO production in isolated rat pancreatic islets requires gene transcription and may lead to inhibition of the Krebs cycle enzyme aconitase. Endocrinology. 1991;129:3167–73. - PubMed
-
- Pavlovic D, Chen MC, Bouwens L et al. Contribution of ductal cells to cytokine responses by human pancreatic islets. Diabetes. 1999;48:29–33. - PubMed
-
- Kroncke KD, Kolb-Bachofen V, Berschick B et al. Activated macrophages kill pancreatic syngeneic islet cells via arginine-dependent NO generation. Biochem Biophys Res Commun. 1991;175:752–8. - PubMed
-
- Steiner L, Kroncke K, Fehsel K et al. Endothelial cells as cytotoxic effector cells: cytokine-activated rat islet endothelial cells lyse syngeneic islet cells via NO. Diabetologia. 1997;40:150–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

