Designer self-assembling peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures
- PMID: 17205123
- PMCID: PMC1762423
- DOI: 10.1371/journal.pone.0000119
Designer self-assembling peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures
Abstract
Biomedical researchers have become increasingly aware of the limitations of conventional 2-dimensional tissue cell culture systems, including coated Petri dishes, multi-well plates and slides, to fully address many critical issues in cell biology, cancer biology and neurobiology, such as the 3-D microenvironment, 3-D gradient diffusion, 3-D cell migration and 3-D cell-cell contact interactions. In order to fully understand how cells behave in the 3-D body, it is important to develop a well-controlled 3-D cell culture system where every single ingredient is known. Here we report the development of a 3-D cell culture system using a designer peptide nanofiber scaffold with mouse adult neural stem cells. We attached several functional motifs, including cell adhesion, differentiation and bone marrow homing motifs, to a self-assembling peptide RADA16 (Ac-RADARADARADARADA-COHN2). These functionalized peptides undergo self-assembly into a nanofiber structure similar to Matrigel. During cell culture, the cells were fully embedded in the 3-D environment of the scaffold. Two of the peptide scaffolds containing bone marrow homing motifs significantly enhanced the neural cell survival without extra soluble growth and neurotrophic factors to the routine cell culture media. In these designer scaffolds, the cell populations with beta-Tubulin(+), GFAP(+) and Nestin(+) markers are similar to those found in cell populations cultured on Matrigel. The gene expression profiling array experiments showed selective gene expression, possibly involved in neural stem cell adhesion and differentiation. Because the synthetic peptides are intrinsically pure and a number of desired function cellular motifs are easy to incorporate, these designer peptide nanofiber scaffolds provide a promising controlled 3-D culture system for diverse tissue cells, and are useful as well for general molecular and cell biology.
Conflict of interest statement
Figures




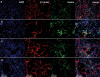


References
-
- Lanza R, Langer R, Vacanti J. Principles of Tissue Engineering, Academic Press; San Diego, USA: 2000. 2nd.
-
- Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, et al. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. - PubMed
-
- Weaver VM, Howlett AR, Langton-Webster B, Petersen OW, Bissell MJ. The development of a functionally relevant cell culture model of progressive human breast cancer. Seminar in Cancer Biol. 1995;6:175–184. - PubMed
-
- Spancake KM, Anderson CB, Weaver VM, Matsunami N, Bissell MJ, et al. E7-transduced human breast epithelial cells show partial differentiation in three-dimensional culture. Cancer Res. 1999;59:6042–6045. - PubMed
-
- Zhau HE, Goodwin TJ, Chang SM, Baker TL, Chung LW. Establishment of a three-dimensional human prostate organoid coculture under microgravity-simulated conditions: evaluation of androgen-induced growth and PSA expression. Cell Dev. Biol. Anim. 1997;33:375–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

