Docking of secretory vesicles is syntaxin dependent
- PMID: 17205130
- PMCID: PMC1762430
- DOI: 10.1371/journal.pone.0000126
Docking of secretory vesicles is syntaxin dependent
Abstract
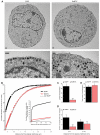
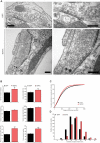
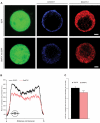
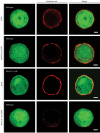
Secretory vesicles dock at the plasma membrane before they undergo fusion. Molecular docking mechanisms are poorly defined but believed to be independent of SNARE proteins. Here, we challenged this hypothesis by acute deletion of the target SNARE, syntaxin, in vertebrate neurons and neuroendocrine cells. Deletion resulted in fusion arrest in both systems. No docking defects were observed in synapses, in line with previous observations. However, a drastic reduction in morphologically docked secretory vesicles was observed in chromaffin cells. Syntaxin-deficient chromaffin cells showed a small reduction in total and plasma membrane staining for the docking factor Munc18-1, which appears insufficient to explain the drastic reduction in docking. The sub-membrane cortical actin network was unaffected by syntaxin deletion. These observations expose a docking role for syntaxin in the neuroendocrine system. Additional layers of regulation may have evolved to make syntaxin redundant for docking in highly specialized systems like synaptic active zones.
Conflict of interest statement
Figures


References
-
- Söllner T, Whiteheart S, Brunner M, Bromage H, Geromanos S, et al. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–323. - PubMed
-
- Ferro-Novick S, Jahn R. Vesicle fusion from yeast to man. Nature. 1994;370:191–193. - PubMed
-
- Hunt JM, Bommert K, Charlton MP, Kistner A, Habermann E, et al. A post-docking role for synaptobrevin in synaptic vesicle fusion. Neuron. 1994;12:1269–1279. - PubMed
-
- Broadie K, Prokop A, Bellen HJ, O'Kane CJ, Schulze KL, et al. Syntaxin and synaptobrevin function downstream of vesicle docking in Drosophila. Neuron. 1995;15:663–673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

