Viral and cellular determinants involved in hepadnaviral entry
- PMID: 17206752
- PMCID: PMC4065874
- DOI: 10.3748/wjg.v13.i1.22
Viral and cellular determinants involved in hepadnaviral entry
Abstract
Hepadnaviridae is a family of hepatotropic DNA viruses that is divided into the genera orthohepadnavirus of mammals and avihepadnavirus of birds. All members of this family can cause acute and chronic hepatic infection, which in the case of human hepatitis B virus (HBV) constitutes a major global health problem. Although our knowledge about the molecular biology of these highly liver-specific viruses has profoundly increased in the last two decades, the mechanisms of attachment and productive entrance into the differentiated host hepatocytes are still enigmatic. The difficulties in studying hepadnaviral entry were primarily caused by the lack of easily accessible in vitro infection systems. Thus, for more than twenty years, differentiated primary hepatocytes from the respective species were the only in vitro models for both orthohepadnaviruses (e.g. HBV) and avihepadnaviruses (e.g. duck hepatitis B virus [DHBV]). Two important discoveries have been made recently regarding HBV: (1) primary hepatocytes from tree-shrews; i.e., Tupaia belangeri, can be substituted for primary human hepatocytes, and (2) a human hepatoma cell line (HepaRG) was established that gains susceptibility for HBV infection upon induction of differentiation in vitro. A number of potential HBV receptor candidates have been described in the past, but none of them have been confirmed to function as a receptor. For DHBV and probably all other avian hepadnaviruses, carboxypeptidase D (CPD) has been shown to be indispensable for infection, although the exact role of this molecule is still under debate. While still restricted to the use of primary duck hepatocytes (PDH), investigations performed with DHBV provided important general concepts on the first steps of hepadnaviral infection. However, with emerging data obtained from the new HBV infection systems, the hope that DHBV utilizes the same mechanism as HBV only partially held true. Nevertheless, both HBV and DHBV in vitro infection systems will help to: (1) functionally dissect the hepadnaviral entry pathways, (2) perform reverse genetics (e.g. test the fitness of escape mutants), (3) titrate and map neutralizing antibodies, (4) improve current vaccines to combat acute and chronic infections of hepatitis B, and (5) develop entry inhibitors for future clinical applications.
Figures
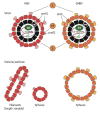

References
-
- Briz V, Poveda E, Soriano V. HIV entry inhibitors: mechanisms of action and resistance pathways. J Antimicrob Chemother. 2006;57:619–627. - PubMed
-
- The International Committee on Taxonomy of Viruses. Available from: http://www.ncbi.nlm.nih.gov/ICTVdb/Ictv/index.htm.
-
- World Health Organization. Available from: http://www.who.int/csr/disease/hepatitis/HepatitisB_whocdscsrlyo2002_2.pdf.
-
- Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–1133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

