A role for Alström syndrome protein, alms1, in kidney ciliogenesis and cellular quiescence
- PMID: 17206865
- PMCID: PMC1761047
- DOI: 10.1371/journal.pgen.0030008
A role for Alström syndrome protein, alms1, in kidney ciliogenesis and cellular quiescence
Abstract
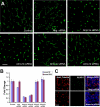
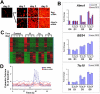
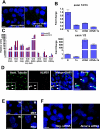
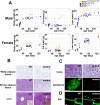
Premature truncation alleles in the ALMS1 gene are a frequent cause of human Alström syndrome. Alström syndrome is a rare disorder characterized by early obesity and sensory impairment, symptoms shared with other genetic diseases affecting proteins of the primary cilium. ALMS1 localizes to centrosomes and ciliary basal bodies, but truncation mutations in Alms1/ALMS1 do not preclude formation of cilia. Here, we show that in vitro knockdown of Alms1 in mice causes stunted cilia on kidney epithelial cells and prevents these cells from increasing calcium influx in response to mechanical stimuli. The stunted-cilium phenotype can be rescued with a 5' fragment of the Alms1 cDNA, which resembles disease-associated alleles. In a mouse model of Alström syndrome, Alms1 protein can be stably expressed from the mutant allele and is required for cilia formation in primary cells. Aged mice developed specific loss of cilia from the kidney proximal tubules, which is associated with foci of apoptosis or proliferation. As renal failure is a common cause of mortality in Alström syndrome patients, we conclude that this disease should be considered as a further example of the class of renal ciliopathies: wild-type or mutant alleles of the Alström syndrome gene can support normal kidney ciliogenesis in vitro and in vivo, but mutant alleles are associated with age-dependent loss of kidney primary cilia.
Conflict of interest statement
Competing interests. RJG owns stock in Phenomix.
Figures
References
-
- Marshall JD, Bronson RT, Collin GB, Nordstrom AD, Maffei P, et al. New Alstrom syndrome phenotypes based on the evaluation of 182 cases. Arch Intern Med. 2005;165:675–683. - PubMed
-
- Singla V, Reiter JF. The primary cilium as the cell's antenna: Signaling at a sensory organelle. Science. 2006;313:629–633. - PubMed
-
- Davis EE, Brueckner M, Katsanis N. The emerging complexity of the vertebrate cilium: New functional roles for an ancient organelle. Dev Cell. 2006;11:9–19. - PubMed
-
- Wilson PD. Polycystic kidney disease: New understanding in the pathogenesis. Int J Biochem Cell Biol. 2004;36:1868–1873. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

