Detection of epithelial to mesenchymal transition in airways of a bleomycin induced pulmonary fibrosis model derived from an alpha-smooth muscle actin-Cre transgenic mouse
- PMID: 17207287
- PMCID: PMC1781437
- DOI: 10.1186/1465-9921-8-1
Detection of epithelial to mesenchymal transition in airways of a bleomycin induced pulmonary fibrosis model derived from an alpha-smooth muscle actin-Cre transgenic mouse
Abstract
Background: Epithelial to mesenchymal transition (EMT) in alveolar epithelial cells (AECs) has been widely observed in patients suffering interstitial pulmonary fibrosis. In vitro studies have also demonstrated that AECs could convert into myofibroblasts following exposure to TGF-beta1. In this study, we examined whether EMT occurs in bleomycin (BLM) induced pulmonary fibrosis, and the involvement of bronchial epithelial cells (BECs) in the EMT. Using an alpha-smooth muscle actin-Cre transgenic mouse (alpha-SMA-Cre/R26R) strain, we labelled myofibroblasts in vivo. We also performed a phenotypic analysis of human BEC lines during TGF-beta1 stimulation in vitro.
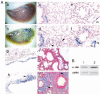
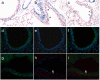
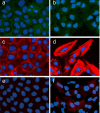
Methods: We generated the alpha-SMA-Cre mouse strain by pronuclear microinjection with a Cre recombinase cDNA driven by the mouse alpha-smooth muscle actin (alpha-SMA) promoter. alpha-SMA-Cre mice were crossed with the Cre-dependent LacZ expressing strain R26R to produce the double transgenic strain alpha-SMA-Cre/R26R. beta-galactosidase (betagal) staining, alpha-SMA and smooth muscle myosin heavy chains immunostaining were carried out simultaneously to confirm the specificity of expression of the transgenic reporter within smooth muscle cells (SMCs) under physiological conditions. BLM-induced peribronchial fibrosis in alpha-SMA-Cre/R26R mice was examined by pulmonary betagal staining and alpha-SMA immunofluorescence staining. To confirm in vivo observations of BECs undergoing EMT, we stimulated human BEC line 16HBE with TGF-beta1 and examined the localization of the myofibroblast markers alpha-SMA and F-actin, and the epithelial marker E-cadherin by immunofluorescence.
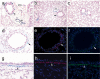
Results: betagal staining in organs of healthy alpha-SMA-Cre/R26R mice corresponded with the distribution of SMCs, as confirmed by alpha-SMA and SM-MHC immunostaining. BLM-treated mice showed significantly enhanced betagal staining in subepithelial areas in bronchi, terminal bronchioles and walls of pulmonary vessels. Some AECs in certain peribronchial areas or even a small subset of BECs were also positively stained, as confirmed by alpha-SMA immunostaining. In vitro, addition of TGF-beta1 to 16HBE cells could also stimulate the expression of alpha-SMA and F-actin, while E-cadherin was decreased, consistent with an EMT.
Conclusion: We observed airway EMT in BLM-induced peribronchial fibrosis mice. BECs, like AECs, have the capacity to undergo EMT and to contribute to mesenchymal expansion in pulmonary fibrosis.
Figures

References
-
- White ES, Atrasz RG, Hu B, Phan SH, Stambolic V, Mak TW, Hogaboam CM, Flaherty KR, Martinez FJ, Kontos CD, et al. Negative regulation of myofibroblast differentiation by PTEN (Phosphatase and Tensin Homolog Deleted on chromosome 10) Am J Respir Crit Care Med. 2006;173(1):112–121. doi: 10.1164/rccm.200507-1058OC. - DOI - PMC - PubMed
-
- Chambers RC, Leoni P, Kaminski N, Laurent GJ, Heller RA. Global expression profiling of fibroblast responses to transforming growth factor-betal reveals the induction of inhibitor of differentiation-1 and provides evidence of smooth muscle cell phenotypic switching. Am J Pathol. 2003;162(2):533–546. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

