Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia
- PMID: 17207473
- PMCID: PMC2204086
- DOI: 10.1016/j.biopsych.2006.10.012
Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia
Abstract
Background: A neonatal ventral hippocampal lesion (NVHL) induces behavioral and physiological anomalies mimicking pathophysiological changes of schizophrenia. Because prefrontal cortical (PFC) pyramidal neurons recorded from adult NVHL rats exhibit abnormal responses to activation of the mesocortical dopaminergic (DA) system, we explored whether these changes are due to an altered DA modulation of pyramidal neurons.
Methods: Whole-cell recordings were used to examine the effects of DA and glutamate agonists on cell excitability in brain slices obtained from pre- (postnatal day [PD] 28-35) and post-pubertal (PD > 61) sham and NVHL animals.
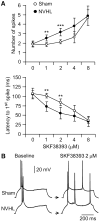
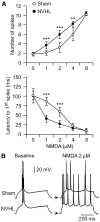
Results: N-methyl d-aspartate (NMDA), alpha-amino-3-hydroxy-5-methylisoxazole propionate (AMPA), and the D(1) agonist SKF38393 increased excitability of deep layer pyramidal neurons in a concentration-dependent manner. The opposite effect was observed with the D(2) agonist quinpirole. The effects of NMDA (but not AMPA) and SKF38393 on cell excitability were significantly higher in slices from NVHL animals, whereas quinpirole decrease of cell excitability was reduced. These differences were not observed in slices from pre-pubertal rats, suggesting that PFC DA and glutamatergic systems become altered after puberty in NVHL rats.
Conclusions: A disruption of PFC dopamine-glutamate interactions might emerge after puberty in brains with an early postnatal deficit in hippocampal inputs, and this disruption could contribute to the manifestation of schizophrenia-like symptoms.
Figures



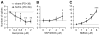

Similar articles
-
Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms.J Neurosci. 2004 Jun 2;24(22):5131-9. doi: 10.1523/JNEUROSCI.1021-04.2004. J Neurosci. 2004. PMID: 15175382 Free PMC article.
-
D(1) dopamine receptors potentiate nmda-mediated excitability increase in layer V prefrontal cortical pyramidal neurons.Cereb Cortex. 2001 May;11(5):452-62. doi: 10.1093/cercor/11.5.452. Cereb Cortex. 2001. PMID: 11313297
-
Dopamine depresses excitatory synaptic transmission onto rat subicular neurons via presynaptic D1-like dopamine receptors.J Neurophysiol. 2000 Jul;84(1):112-9. doi: 10.1152/jn.2000.84.1.112. J Neurophysiol. 2000. PMID: 10899189
-
The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia.Behav Brain Res. 2009 Dec 7;204(2):295-305. doi: 10.1016/j.bbr.2008.11.039. Epub 2008 Dec 3. Behav Brain Res. 2009. PMID: 19100784 Free PMC article. Review.
-
Hippocampal dysfunction and disruption of dopamine system regulation in an animal model of schizophrenia.Neurotox Res. 2008 Oct;14(2-3):97-104. doi: 10.1007/BF03033801. Neurotox Res. 2008. PMID: 19073417 Free PMC article. Review.
Cited by
-
Cortical-striatal gene expression in neonatal hippocampal lesion (NVHL)-amplified cocaine sensitization.Genes Brain Behav. 2013 Jul;12(5):564-75. doi: 10.1111/gbb.12051. Epub 2013 Jun 22. Genes Brain Behav. 2013. PMID: 23682998 Free PMC article.
-
Differential contributions of prefrontal and hippocampal dopamine D(1) and D(2) receptors in human cognitive functions.J Neurosci. 2008 Nov 12;28(46):12032-8. doi: 10.1523/JNEUROSCI.3446-08.2008. J Neurosci. 2008. PMID: 19005068 Free PMC article.
-
Differences between adolescents and adults in the acute effects of PCP and ketamine and in sensitization following intermittent administration.Pharmacol Biochem Behav. 2017 Jun;157:24-34. doi: 10.1016/j.pbb.2017.04.007. Epub 2017 Apr 22. Pharmacol Biochem Behav. 2017. PMID: 28442368 Free PMC article.
-
Concurrent upregulation of postsynaptic L-type Ca(2+) channel function and protein kinase A signaling is required for the periadolescent facilitation of Ca(2+) plateau potentials and dopamine D1 receptor modulation in the prefrontal cortex.Neuropharmacology. 2011 May;60(6):953-62. doi: 10.1016/j.neuropharm.2011.01.041. Epub 2011 Feb 1. Neuropharmacology. 2011. PMID: 21288471 Free PMC article.
-
Altered basolateral amygdala encoding in an animal model of schizophrenia.J Neurosci. 2015 Apr 22;35(16):6394-400. doi: 10.1523/JNEUROSCI.5096-14.2015. J Neurosci. 2015. PMID: 25904791 Free PMC article.
References
-
- Becker A, Grecksch G, Bernstein HG, Hollt V, Bogerts B. Social behaviour in rats lesioned with ibotenic acid in the hippocampus: quantitative and qualitative analysis. Psychopharmacology (Berl) 1999;144:333–338. - PubMed
-
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078 –1092. - PubMed
-
- Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B, et al. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry. 2003a;160:709 –719. - PubMed
-
- Callicott JH, Mattay VS, Bertolino A, Finn K, Jones K, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20 –26. - PubMed
-
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: More than up or down. Am J Psychiatry. 2003b;160:2209 –2215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

