Role of retinoic acid during forebrain development begins late when Raldh3 generates retinoic acid in the ventral subventricular zone
- PMID: 17207476
- PMCID: PMC1994967
- DOI: 10.1016/j.ydbio.2006.11.035
Role of retinoic acid during forebrain development begins late when Raldh3 generates retinoic acid in the ventral subventricular zone
Abstract
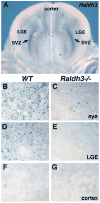
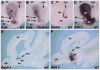
Retinoic acid (RA) synthesized by Raldh3 in the frontonasal surface ectoderm of chick embryos has been suggested to function in early forebrain patterning by regulating Fgf8, Shh, and Meis2 expression. Similar expression of Raldh3 exists in E8.75 mouse embryos, but Raldh2 is also expressed in the optic vesicle at this stage suggesting that both genes may play a role in early forebrain patterning. Furthermore, Raldh3 is expressed later in the forebrain itself (lateral ganglionic eminence; LGE) starting at E12.5, suggesting a later role in forebrain neurogenesis. Here we have analyzed mouse embryos carrying single or double null mutations in Raldh2 and Raldh3 for defects in forebrain development. Raldh2(-/-);Raldh3(-/-) embryos completely lacked RA signaling activity in the early forebrain, but exhibited relatively normal expression of Fgf8, Shh, and Meis2 in the forebrain. Thus, we find no clear requirement for RA in controlling expression of these important forebrain patterning genes, but Raldh3 expression in the frontonasal surface ectoderm was found to be needed for normal Fgf8 expression in the olfactory pit. Our studies revealed that later expression of Raldh3 in the subventricular zone of the LGE is required for RA signaling activity in the ventral forebrain. Importantly, expression of dopamine receptor D2 in E18.5 Raldh3(-/-) embryos was essentially eliminated in the developing nucleus accumbens, a tissue lying close to the source of RA provided by Raldh3. Our results suggest that the role of RA during forebrain development begins late when Raldh3 expression initiates in the ventral subventricular zone.
Figures




Similar articles
-
Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice.Development. 2002 May;129(9):2271-82. doi: 10.1242/dev.129.9.2271. Development. 2002. PMID: 11959834 Free PMC article.
-
Retinaldehyde dehydrogenase 2 (RALDH2)- independent patterns of retinoic acid synthesis in the mouse embryo.Proc Natl Acad Sci U S A. 2002 Dec 10;99(25):16111-6. doi: 10.1073/pnas.252626599. Epub 2002 Nov 26. Proc Natl Acad Sci U S A. 2002. PMID: 12454286 Free PMC article.
-
Raldh2 expression in optic vesicle generates a retinoic acid signal needed for invagination of retina during optic cup formation.Dev Dyn. 2004 Oct;231(2):270-7. doi: 10.1002/dvdy.20128. Dev Dyn. 2004. PMID: 15366004
-
Retinoic acid regulation of the somitogenesis clock.Birth Defects Res C Embryo Today. 2007 Jun;81(2):84-92. doi: 10.1002/bdrc.20092. Birth Defects Res C Embryo Today. 2007. PMID: 17600781 Free PMC article. Review.
-
Emerging roles for retinoids in regeneration and differentiation in normal and disease states.Biochim Biophys Acta. 2012 Jan;1821(1):213-21. doi: 10.1016/j.bbalip.2011.08.002. Epub 2011 Aug 7. Biochim Biophys Acta. 2012. PMID: 21855651 Free PMC article. Review.
Cited by
-
Retinoic acid signaling pathways.Development. 2019 Jul 4;146(13):dev167502. doi: 10.1242/dev.167502. Development. 2019. PMID: 31273085 Free PMC article. Review.
-
Regulating Retinoic Acid Availability during Development and Regeneration: The Role of the CYP26 Enzymes.J Dev Biol. 2020 Mar 5;8(1):6. doi: 10.3390/jdb8010006. J Dev Biol. 2020. PMID: 32151018 Free PMC article. Review.
-
Dlx1/2-dependent expression of Meis2 promotes neuronal fate determination in the mammalian striatum.Development. 2022 Feb 15;149(4):dev200035. doi: 10.1242/dev.200035. Epub 2022 Feb 23. Development. 2022. PMID: 35156680 Free PMC article.
-
Modular patterning of structure and function of the striatum by retinoid receptor signaling.Proc Natl Acad Sci U S A. 2008 May 6;105(18):6765-70. doi: 10.1073/pnas.0802109105. Epub 2008 Apr 28. Proc Natl Acad Sci U S A. 2008. PMID: 18443282 Free PMC article.
-
Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily.Expert Opin Drug Metab Toxicol. 2008 Jun;4(6):697-720. doi: 10.1517/17425255.4.6.697. Expert Opin Drug Metab Toxicol. 2008. PMID: 18611112 Free PMC article. Review.
References
-
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. - PubMed
-
- Blentic A, Gale E, Maden M. Retinoic acid signalling centres in the avian embryo identified by sites of expression of synthesising and catabolising enzymes. Dev Dyn. 2003;227:114–127. - PubMed
-
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: Opening the X-files. Science. 2001;294:1866–1870. - PubMed
-
- Corbin JG, Gaiano N, Machold RP, Langston A, Fishell G. The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development. 2000;127:5007–5020. - PubMed
-
- Del Corral RD, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

