Novel animal models for Sjögren's syndrome: expression and transfer of salivary gland dysfunction from regulatory T cell-deficient mice
- PMID: 17207605
- PMCID: PMC3970716
- DOI: 10.1016/j.jaut.2006.11.003
Novel animal models for Sjögren's syndrome: expression and transfer of salivary gland dysfunction from regulatory T cell-deficient mice
Abstract
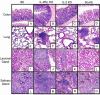
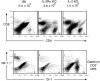
IL-2 knockout (KO), IL-2Ralpha KO and scurfy mice lack the CD4+CD25+ regulatory T (Treg) cells and develop severe inflammation in multiple organs, although organs affected vary among these strains. We asked if salivary and lacrimal glands, the main organs affected in Sjögren's syndrome, are targeted in these strains. Severe lymphocyte and neutrophil infiltration in the salivary and lacrimal glands and a decrease in salivary secretory function were observed in IL-2 KO and IL-2Ralpha KO mice, but not in scurfy mice. Interestingly, transfer of lymph node cells from scurfy mice to RAG-1 KO recipients rapidly and effectively induced inflammation and loss of function in the salivary glands. Furthermore, we observed that daily LPS feeding in scurfy mice also induced inflammation in the salivary glands. Our study demonstrates several novel models for Sjögren's syndrome, including an adoptive transfer model that shows that scurfy mice have dormant salivary gland-specific autoreactive lymphocytes that can be activated by certain environmental factors, such as those present in RAG-1 KO mice.
Figures




Similar articles
-
Altered characteristics of regulatory T cells in target tissues of Sjögren's syndrome in murine models.Mol Immunol. 2024 Oct;174:47-56. doi: 10.1016/j.molimm.2024.08.003. Epub 2024 Aug 27. Mol Immunol. 2024. PMID: 39197397
-
B7-H4 Inhibits the Development of Primary Sjögren's Syndrome by Regulating Treg Differentiation in NOD/Ltj Mice.J Immunol Res. 2020 Sep 27;2020:4896727. doi: 10.1155/2020/4896727. eCollection 2020. J Immunol Res. 2020. PMID: 33062721 Free PMC article.
-
CD8 T cells contribute to lacrimal gland pathology in the nonobese diabetic mouse model of Sjögren syndrome.Immunol Cell Biol. 2017 Sep;95(8):684-694. doi: 10.1038/icb.2017.38. Epub 2017 May 3. Immunol Cell Biol. 2017. PMID: 28465508 Free PMC article.
-
Pathogenesis of Sjögren's syndrome-like autoimmune lesions in MRL/lpr mice.Pathol Int. 1994 Aug;44(8):559-68. doi: 10.1111/j.1440-1827.1994.tb01716.x. Pathol Int. 1994. PMID: 7952145 Review.
-
The complexity of Sjögren's syndrome: novel aspects on pathogenesis.Immunol Lett. 2011 Dec 30;141(1):1-9. doi: 10.1016/j.imlet.2011.06.007. Epub 2011 Jul 12. Immunol Lett. 2011. PMID: 21777618 Review.
Cited by
-
Ocular surface disease and dacryoadenitis in aging C57BL/6 mice.Am J Pathol. 2014 Mar;184(3):631-43. doi: 10.1016/j.ajpath.2013.11.019. Epub 2014 Jan 3. Am J Pathol. 2014. PMID: 24389165 Free PMC article.
-
A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells.Immunity. 2009 Feb 20;30(2):204-17. doi: 10.1016/j.immuni.2008.11.014. Epub 2009 Jan 29. Immunity. 2009. PMID: 19185518 Free PMC article.
-
Aged regulatory T cells fail to control autoimmune lacrimal gland pathogenic CD4+ T cells.Geroscience. 2025 Jun;47(3):4219-4240. doi: 10.1007/s11357-025-01576-y. Epub 2025 Mar 7. Geroscience. 2025. PMID: 40053297 Free PMC article.
-
Etiopathogenesis of primary biliary cirrhosis.World J Gastroenterol. 2008 Jun 7;14(21):3328-37. doi: 10.3748/wjg.14.3328. World J Gastroenterol. 2008. PMID: 18528930 Free PMC article. Review.
-
Spontaneous autoimmune dacryoadenitis in aged CD25KO mice.Am J Pathol. 2010 Aug;177(2):744-53. doi: 10.2353/ajpath.2010.091116. Epub 2010 Jun 21. Am J Pathol. 2010. PMID: 20566743 Free PMC article.
References
-
- Soliotis FC, Moutsopoulos HM. Sjögren's syndrome. Autoimmunity. 2004;37:305–307. - PubMed
-
- Hansen A, Lipsky PE, Dorner T. New concepts in the pathogenesis of Sjögren syndrome: many questions, fewer answers. Curr Opin Rheumatol. 2003;15:563–570. - PubMed
-
- Borchers AT, Naguwa SM, Keen CL, Gershwin ME. Immunopathogenesis of Sjögren's syndrome. Clin Rev Allergy Immunol. 2003;25:89–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR-051203/AR/NIAMS NIH HHS/United States
- AR047988/AR/NIAMS NIH HHS/United States
- R01 AR044719/AR/NIAMS NIH HHS/United States
- R01 AR049449/AR/NIAMS NIH HHS/United States
- R01 AR047988/AR/NIAMS NIH HHS/United States
- AI-36938/AI/NIAID NIH HHS/United States
- AR045222/AR/NIAMS NIH HHS/United States
- K23-DK59850/DK/NIDDK NIH HHS/United States
- P50 AR045222/AR/NIAMS NIH HHS/United States
- R01 AR051203/AR/NIAMS NIH HHS/United States
- DE-017579/DE/NIDCR NIH HHS/United States
- R01 AI036938/AI/NIAID NIH HHS/United States
- AR049449/AR/NIAMS NIH HHS/United States
- K23 DK059850/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
