Effects of DksA, GreA, and GreB on transcription initiation: insights into the mechanisms of factors that bind in the secondary channel of RNA polymerase
- PMID: 17207814
- PMCID: PMC1839928
- DOI: 10.1016/j.jmb.2006.12.013
Effects of DksA, GreA, and GreB on transcription initiation: insights into the mechanisms of factors that bind in the secondary channel of RNA polymerase
Abstract
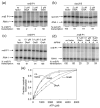
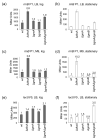
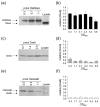
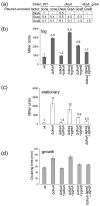
Escherichia coli DksA, GreA, and GreB have similar structures and bind to the same location on RNA polymerase (RNAP), the secondary channel. We show that GreB can fulfil some roles of DksA in vitro, including shifting the promoter-open complex equilibrium in the dissociation direction, thus allowing rRNA promoters to respond to changes in the concentration of ppGpp and NTPs. However, unlike deletion of the dksA gene, deletion of greB had no effect on rRNA promoters in vivo. We show that the apparent affinities of DksA and GreB for RNAP are similar, but the cellular concentration of GreB is much lower than that of DksA. When over-expressed and in the absence of competing GreA, GreB almost completely complemented the loss of dksA in control of rRNA expression, indicating its inability to regulate rRNA transcription in vivo results primarily from its low concentration. In contrast to GreB, the apparent affinity of GreA for RNAP was weaker than that of DksA, GreA affected rRNA promoters only modestly in vitro and, even when over-expressed, GreA did not affect rRNA transcription in vivo. Thus, binding in the secondary channel is necessary but insufficient to explain the effect of DksA on rRNA transcription. Neither Gre factor was capable of fulfilling two other functions of DksA in transcription initiation: co-activation of amino acid biosynthetic gene promoters with ppGpp and compensation for the loss of the omega subunit of RNAP in the response of rRNA promoters to ppGpp. Our results provide important clues to the mechanisms of both negative and positive control of transcription initiation by DksA.
Figures

Similar articles
-
Antagonistic regulation of Escherichia coli ribosomal RNA rrnB P1 promoter activity by GreA and DksA.J Biol Chem. 2006 Jun 2;281(22):15238-48. doi: 10.1074/jbc.M601531200. Epub 2006 Apr 5. J Biol Chem. 2006. PMID: 16597620
-
Effects on growth by changes of the balance between GreA, GreB, and DksA suggest mutual competition and functional redundancy in Escherichia coli.J Bacteriol. 2012 Jan;194(2):261-73. doi: 10.1128/JB.06238-11. Epub 2011 Nov 4. J Bacteriol. 2012. PMID: 22056927 Free PMC article.
-
Delayed inhibition mechanism for secondary channel factor regulation of ribosomal RNA transcription.Elife. 2019 Feb 5;8:e40576. doi: 10.7554/eLife.40576. Elife. 2019. PMID: 30720429 Free PMC article.
-
rRNA transcription in Escherichia coli.Annu Rev Genet. 2004;38:749-70. doi: 10.1146/annurev.genet.38.072902.091347. Annu Rev Genet. 2004. PMID: 15568992 Review.
-
[Mechanisms of action of RNA polymerase-binding transcription factors that do not bind to DNA].Biofizika. 2009 Sep-Oct;54(5):773-90. Biofizika. 2009. PMID: 19894614 Review. Russian.
Cited by
-
The δ subunit of RNA polymerase is required for rapid changes in gene expression and competitive fitness of the cell.J Bacteriol. 2013 Jun;195(11):2603-11. doi: 10.1128/JB.00188-13. Epub 2013 Mar 29. J Bacteriol. 2013. PMID: 23543716 Free PMC article.
-
CdnL, a member of the large CarD-like family of bacterial proteins, is vital for Myxococcus xanthus and differs functionally from the global transcriptional regulator CarD.Nucleic Acids Res. 2010 Aug;38(14):4586-98. doi: 10.1093/nar/gkq214. Epub 2010 Apr 5. Nucleic Acids Res. 2010. PMID: 20371514 Free PMC article.
-
DksA-RNA polymerase interactions support new origin formation and DNA repair in Escherichia coli.Mol Microbiol. 2019 May;111(5):1382-1397. doi: 10.1111/mmi.14227. Epub 2019 Mar 22. Mol Microbiol. 2019. PMID: 30779388 Free PMC article.
-
Mycobacterial RNA polymerase forms unstable open promoter complexes that are stabilized by CarD.Nucleic Acids Res. 2015 Jan;43(1):433-45. doi: 10.1093/nar/gku1231. Epub 2014 Dec 15. Nucleic Acids Res. 2015. PMID: 25510492 Free PMC article.
-
Control of transcription elongation by GreA determines rate of gene expression in Streptococcus pneumoniae.Nucleic Acids Res. 2014;42(17):10987-99. doi: 10.1093/nar/gku790. Epub 2014 Sep 4. Nucleic Acids Res. 2014. PMID: 25190458 Free PMC article.
References
-
- Record MT, Jr, Reznikoff WS, Craig ML, McQuade KL, Schlaz PJ. Escherichia coli RNA polymerase (Eσ70), promoters, and the kinetics of the steps of transcription initiation. In: Neidhardt FC, editor. Escherichia coli and Salmonella. ASM Press; Washington, D.C: 1996. pp. 792–820.
-
- Paul BJ, Ross W, Gaal T, Gourse RL. rRNA transcription in Escherichia coli. Annu Rev Genet. 2004;28:749–770. - PubMed
-
- Haugen SP, Berkman MB, Ross W, Gaal T, Ward C, Gourse RL. rRNA promoter regulation by nonoptimal binding of σ region 1.2: an additional recognition element for RNA polymerase. Cell. 2006;125:1069–1082. - PubMed
-
- Murray HD, Schneider DA, Gourse RL. Control of rRNA expression by small molecules is dynamic and nonredundant. Mol Cell. 2003;12:125–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

