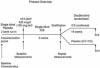
The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial
- PMID: 17208587
- PMCID: PMC2872157
- DOI: 10.1016/j.jaci.2006.10.035
The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial
Abstract
Background: Although guidelines recommend anti-inflammatory therapy for persistent asthma, recent studies suggest that 25% to 35% of patients with asthma may not improve lung function with inhaled corticosteroids.
Objective: To evaluate potential biomarkers of predicting short-term (6-week) response to inhaled corticosteroid with subsequent evaluation of responders and nonresponders to asthma control over a longer interval (16 additional weeks).
Methods: Eighty-three subjects with asthma off steroid were enrolled in this multicenter study. Biomarkers and asthma characteristics were evaluated as predictors of inhaled corticosteroid response over a 6-week trial for changes in FEV(1) and methacholine PC(20). After this, an additional 4-month trial evaluated asthma control.
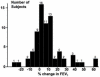
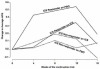
Results: Although multiple baseline predictors had significant correlations with improvements for short-term inhaled steroid success, the only strong correlations (r >or= +/- 0.6) were albuterol reversibility (r = 0.83; P < .001), FEV(1)/forced vital capacity (r = -0.75; P < .001), and FEV(1) % predicted (r = -0.71; P < .001). Dividing the subjects in the short-term inhaled steroid trial into responders (>5% FEV(1) improvement) and nonresponders (<or=5%) determined the longer-term need for steroids. For the nonresponders, asthma control remained unchanged whether inhaled corticosteroids were continued or were substituted with a placebo (P = .99). The good short-term responders maintained asthma control longer-term only if maintained on inhaled steroids (P = .007).
Conclusion: The short-term response to inhaled corticosteroids with regard to FEV(1) improvement predicts long-term asthma control.
Clinical implications: The decision to use long-term inhaled steroids could be based on a short-term trial. Different therapeutic strategies would need to be established for nonresponders.
Figures

References
-
- Barnes PJ, Pedersen S, Busse WW. Efficacy and safety of inhaled corticosteroids. New developments. Am J Respir Crit Care Med. 1998;157(3 Pt 2):S1–53. - PubMed
-
- Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 Pt 2):S17–22. - PubMed
-
- National Asthma Education and Prevention Program . Guidelines for the diagnosis and management of asthma. National Institutes of Health/National Heart, Lung, and Blood Institute; Bethesda (MD): 1997. pp. 1–146. Expert Panel Report 2. Publication no. 97-4051.
-
- Global Initiative for Asthma . Global strategy for asthma management and prevention. National Institutes of Health/National Heart, Lung, and Blood Institute; Bethesda (MD): 1995. NHLBI/NIH Workshop Report. Publication no. 95-3659.
-
- Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Pineiro A, Wei LX, et al. Montelukast/Beclomethasone Study Group Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Ann Intern Med. 1999;130(6):487–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 HL051843/HL/NHLBI NIH HHS/United States
- HL051834/HL/NHLBI NIH HHS/United States
- HL051823/HL/NHLBI NIH HHS/United States
- HL04285/HL/NHLBI NIH HHS/United States
- U10 HL051823/HL/NHLBI NIH HHS/United States
- U10 HL051834/HL/NHLBI NIH HHS/United States
- U10 HL074073/HL/NHLBI NIH HHS/United States
- HL051843/HL/NHLBI NIH HHS/United States
- HL051810/HL/NHLBI NIH HHS/United States
- K23 HL004285/HL/NHLBI NIH HHS/United States
- HL051845/HL/NHLBI NIH HHS/United States
- HL051831/HL/NHLBI NIH HHS/United States
- U10 HL051831/HL/NHLBI NIH HHS/United States
- U10 HL051845/HL/NHLBI NIH HHS/United States
- HL056443/HL/NHLBI NIH HHS/United States
- U10 HL056443/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

