Resolving the fluorescence response of Escherichia coli carbamoyl phosphate synthetase: mapping intra- and intersubunit conformational changes
- PMID: 17209549
- PMCID: PMC2559813
- DOI: 10.1021/bi061642n
Resolving the fluorescence response of Escherichia coli carbamoyl phosphate synthetase: mapping intra- and intersubunit conformational changes
Abstract
Carbamoyl phosphate synthetase (CPS) from Escherichia coli is potentially overlaid with a network of allosterism, interconnecting active sites, effector binding sites, and aggregate interfaces to control its mechanisms of catalytic synchronization, regulation, and oligomerization, respectively. To characterize these conformational changes, a tryptophan-free variant of CPS was genetically engineered by substituting six native tryptophans with tyrosines. Each tryptophan was then reinserted, singly, as a specific fluorescence probe of its corresponding microenvironment. The amino acid substitutions themselves result in little apparent disruption of the protein; variants maintain catalytic and allosteric functionality, and the fluorescence properties of each tryptophan, while unique, are additive to wild-type CPS. Whereas the collective, intrinsic fluorescence response of E. coli CPS is largely insensitive to ligand binding, changes of the individual probes in intensity, lifetime, anisotropy, and accessibility to acrylamide quenching highlight the dynamic interplay between several protein domains, as well as between subunits. W213 within the carboxy phosphate domain, for example, exhibits an almost 40% increase in intensity upon saturation with ATP; W437 of the oligomerization domain, in contrast, is essentially silent in its fluorescence to the binding of ligands. Nucleotide and bicarbonate association within the large subunit induces fluorescence changes in both W170 and W175 of the small subunit, indicative of the type of long-range interactions purportedly synchronizing the carboxy phosphate and amidotransferase domains of the enzyme to initiate catalysis. ATP and ADP engender different fluorescence responses in most tryptophans, perhaps reflecting coordinating, conformational changes accompanying the cycling of reactants and products during catalysis.
Figures




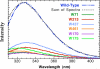
 , W71
, W71  , W213
, W213  , W437
, W437  , W461
, W461  , W170
, W170  , and W175
, and W175  .
.
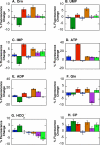
 , W71
, W71  , W213
, W213  , W437
, W437  , W461
, W461  , W170
, W170  , and W175
, and W175  .
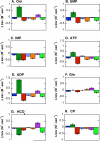
.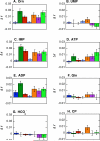
 , W71
, W71  , W213
, W213  , W437
, W437  , W461
, W461  , W170
, W170  , and W175
, and W175  .
.Similar articles
-
Allosteric control of the oligomerization of carbamoyl phosphate synthetase from Escherichia coli.Biochemistry. 2001 Sep 18;40(37):11030-6. doi: 10.1021/bi011121u. Biochemistry. 2001. PMID: 11551199
-
Regulatory changes in the control of carbamoyl phosphate synthetase induced by truncation and mutagenesis of the allosteric binding domain.Biochemistry. 1995 Oct 24;34(42):13920-7. doi: 10.1021/bi00042a025. Biochemistry. 1995. PMID: 7577987
-
Function of the major synthetase subdomains of carbamyl-phosphate synthetase.J Biol Chem. 1996 Jun 7;271(23):13762-9. doi: 10.1074/jbc.271.23.13762. J Biol Chem. 1996. PMID: 8662713
-
Carbamoyl phosphate synthetase: a crooked path from substrates to products.Curr Opin Chem Biol. 1998 Oct;2(5):624-32. doi: 10.1016/s1367-5931(98)80094-x. Curr Opin Chem Biol. 1998. PMID: 9818189 Review.
-
The amidotransferase family of enzymes: molecular machines for the production and delivery of ammonia.Biochemistry. 1999 Jun 22;38(25):7891-9. doi: 10.1021/bi990871p. Biochemistry. 1999. PMID: 10387030 Review.
Cited by
-
Substrate activation and conformational dynamics of guanosine 5'-monophosphate synthetase.Biochemistry. 2013 Aug 6;52(31):5225-35. doi: 10.1021/bi3017075. Epub 2013 Jul 23. Biochemistry. 2013. PMID: 23841499 Free PMC article.
-
Near IR heptamethine cyanine dye-mediated cancer imaging.Clin Cancer Res. 2010 May 15;16(10):2833-44. doi: 10.1158/1078-0432.CCR-10-0059. Epub 2010 Apr 21. Clin Cancer Res. 2010. PMID: 20410058 Free PMC article.
-
Fluorescence spectroscopy as a probe of the effect of phosphorylation at serine 40 of tyrosine hydroxylase on the conformation of its regulatory domain.Biochemistry. 2011 Mar 29;50(12):2364-70. doi: 10.1021/bi101844p. Epub 2011 Feb 22. Biochemistry. 2011. PMID: 21302933 Free PMC article.
-
Diffusional channeling in the sulfate-activating complex: combined continuum modeling and coarse-grained brownian dynamics studies.Biophys J. 2008 Nov 15;95(10):4659-67. doi: 10.1529/biophysj.108.140038. Epub 2008 Aug 8. Biophys J. 2008. PMID: 18689458 Free PMC article.
References
-
- Anderson PM, Meister A. Evidence for an activated form of carbon dioxide in the reaction catalyzed by Escherichia coli carbamyl phosphate synthetase. Biochemistry. 1965;4:2803–2809. - PubMed
-
- Meister A. Mechanism and regulation of the glutamine-dependent carbamoyl phosphate synthetase of E. coli. Adv. Enzymol. 1989;62:315–374. - PubMed
-
- Thoden JB, Holden HM, Wesenberg G, Raushel FM, Rayment I. Structure of carbamoyl phosphate synthetase: a journey of 96Å from substrate to product. Biochemistry. 1997;36:6305–6316. - PubMed
-
- Holden HM, Thoden JB, Raushel FM. Carbamoyl phosphate synthetase: a tunnel runs through it. Current Opinion in Structural Biol. 1998;8:679–685. - PubMed
-
- Raushel FM, Thoden JB, Reinhart GD, Holden H,M. Carbamoyl phosphate synthetase: a crooked path from substrates to products. Current Opinion in Chem. Biol. 1998;2:624–632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

