Oxyferryl heme and not tyrosyl radical is the likely culprit in prostaglandin H synthase-1 peroxidase inactivation
- PMID: 17209563
- PMCID: PMC2851183
- DOI: 10.1021/bi061859h
Oxyferryl heme and not tyrosyl radical is the likely culprit in prostaglandin H synthase-1 peroxidase inactivation
Abstract
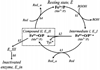
Prostaglandin H synthase-1 (PGHS-1) is a bifunctional heme protein catalyzing both a peroxidase reaction, in which peroxides are converted to alcohols, and a cyclooxygenase reaction, in which arachidonic acid is converted into prostaglandin G2. Reaction of PGHS-1 with peroxide forms Intermediate I, which has an oxyferryl heme and a porphyrin radical. An intramolecular electron transfer from Tyr385 to Intermediate I forms Intermediate II, which contains two oxidants: an oxyferryl heme and the Tyr385 radical required for cyclooxygenase catalysis. Self-inactivation of the peroxidase begins with Intermediate II, but it has been unclear which of the two oxidants is involved. The kinetics of tyrosyl radical, oxyferryl heme, and peroxidase inactivation were examined in reactions of PGHS-1 reconstituted with heme or mangano protoporphyrin IX with a lipid hydroperoxide, 15-hydroperoxyeicosatetraenoic acid (15-HPETE), and ethyl hydrogen peroxide (EtOOH). Tyrosyl radical formation was significantly faster with 15-HPETE than with EtOOH and roughly paralleled oxyferryl heme formation at low peroxide levels. However, the oxyferryl heme intensity decayed much more rapidly than the tyrosyl radical intensity at high peroxide levels. The rates of reactions for PGHS-1 reconstituted with MnPPIX were approximately an order of magnitude slower, and the initial species formed displayed a wide singlet (WS) radical, rather than the wide doublet radical observed with PGHS-1 reconstituted with heme. Inactivation of the peroxidase activity during the reaction of PGHS-1 with EtOOH or 15-HPETE correlated with oxyferryl heme decay, but not with changes in tyrosyl radical intensity or EPR line shape, indicating that the oxyferryl heme, and not the tyrosyl radical, is responsible for the self-destructive peroxidase side reactions. Computer modeling to a minimal mechanism was consistent with oxyferryl heme being the source of peroxidase inactivation.
Figures








References
-
- Rouzer CA, Marnett LJ. Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem. Rev. 2003;103:2239–2304. - PubMed
-
- Tsai A-L, Kulmacz RJ. Tyrosyl radicals in prostaglandin H synthase-1 and -2. Prostaglandins Leukotrienes Med. 2000;62:231–254. - PubMed
-
- Lambeir AM, Markey CM, Dunford HB, Marnett LJ. Spectral properties of the higher oxidation states of prostaglandin H synthase. J. Biol. Chem. 1985;260:14894–14896. - PubMed
-
- Dietz R, Nastainczyk W, Ruf HH. Higher oxidation states of prostaglandin H synthase. Rapid electronic spectroscopy detected two spectral intermediates during the peroxidase reaction with prostaglandin G2. Eur. J. Biochem. 1988;171:321–328. - PubMed
-
- Shimokawa T, Kulmacz RJ, DeWitt DL, Smith WL. Tyrosine 385 of prostaglandin endoperoxide synthase is required for cyclooxygenase catalysis. J. Biol. Chem. 1990;265:20073–20076. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

