Probing DNA bulges with designed helical spirocyclic molecules
- PMID: 17209566
- PMCID: PMC2569198
- DOI: 10.1021/bi061744d
Probing DNA bulges with designed helical spirocyclic molecules
Abstract
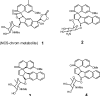
Because bulged structures (unpaired bases) in nucleic acids are of general biological significance, it has been of interest to design small molecules as specific probes of bulge function. On the basis of our earlier work with the specific DNA bulge-binding metabolite obtained from the enediyne antitumor antibiotic neocarzinostatin chromophore (NCS-chrom), we have prepared three small helical spirocyclic molecules that most closely mimic the natural product. These wedge-shaped molecules resemble the natural product in having the sugar residue attached to the same five-membered ring system. In one instance, the sugar is aminoglucose in beta-glycosidic linkage, and in the other, two enantiomers have the natural sugar N-methylfucosamine in alpha-glycosidic linkage. All three analogues were found to interfere with bulge-specific cleavage by NCS-chrom and the ability of bulged DNA to serve as a template for DNA polymerase 1 in accordance with their binding affinities for DNA containing a two-base bulge. Comparable results were obtained with the analogues for the less efficiently cleaved three-base bulge DNA structures. In each situation, the enantiomers possessing the natural sugar in alpha-glycosidic linkage are the most potent inhibitors of the cleavage reaction. In the DNA polymerase reactions, again, the closest natural product mimics were the most effective in selectively impeding nucleotide extension at the bulge site, presumably by complex formation. These results demonstrate the potential usefulness of bulge-binding compounds in modifying DNA structure and function and support efforts to design and prepare reactive species of these molecules that can covalently modify bulged DNA.
Figures

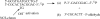






Similar articles
-
Solution structure of a designed spirocyclic helical ligand binding at a two-base bulge site in DNA.Biochemistry. 2007 Apr 24;46(16):4793-803. doi: 10.1021/bi602599d. Epub 2007 Mar 28. Biochemistry. 2007. PMID: 17388570 Free PMC article.
-
Stereochemical control of small molecule binding to bulged DNA: comparison of structures of spirocyclic enantiomer-bulged DNA complexes.Biochemistry. 2004 Jan 27;43(3):641-50. doi: 10.1021/bi035824i. Biochemistry. 2004. PMID: 14730968
-
New complex of post-activated neocarzinostatin chromophore with DNA: bulge DNA binding from the minor groove.Biochemistry. 2003 Feb 11;42(5):1186-98. doi: 10.1021/bi0206210. Biochemistry. 2003. PMID: 12564921
-
Advances in Spirocyclic Hybrids: Chemistry and Medicinal Actions.Curr Med Chem. 2018;25(31):3748-3767. doi: 10.2174/0929867325666180309124821. Curr Med Chem. 2018. PMID: 29521213 Review.
-
Battle for the bulge: directing small molecules to DNA and RNA defects.Chem Biol. 2002 Aug;9(8):854-5. doi: 10.1016/s1074-5521(02)00197-7. Chem Biol. 2002. PMID: 12204683 Review.
Cited by
-
DNA damage-site recognition by lysine conjugates.Proc Natl Acad Sci U S A. 2007 Aug 7;104(32):13016-21. doi: 10.1073/pnas.0705701104. Epub 2007 Jul 30. Proc Natl Acad Sci U S A. 2007. PMID: 17664419 Free PMC article.
-
Designed DNA probes from the neocarzinostatin family: impact of glycosyl linkage stereochemistry on bulge base binding.Bioorg Med Chem. 2009 Mar 15;17(6):2428-32. doi: 10.1016/j.bmc.2009.02.005. Epub 2009 Feb 10. Bioorg Med Chem. 2009. PMID: 19243952 Free PMC article.
-
Solution structure of a designed spirocyclic helical ligand binding at a two-base bulge site in DNA.Biochemistry. 2007 Apr 24;46(16):4793-803. doi: 10.1021/bi602599d. Epub 2007 Mar 28. Biochemistry. 2007. PMID: 17388570 Free PMC article.
-
Small molecule ligands for bulged RNA secondary structures.Org Lett. 2009 Sep 17;11(18):4052-5. doi: 10.1021/ol901478x. Org Lett. 2009. PMID: 19678613 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

