Postnatal development of cannabinoid receptor type 1 expression in rodent somatosensory cortex
- PMID: 17210229
- PMCID: PMC1850104
- DOI: 10.1016/j.neuroscience.2006.11.033
Postnatal development of cannabinoid receptor type 1 expression in rodent somatosensory cortex
Abstract
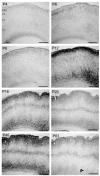
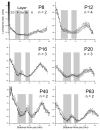
Endocannabinoids are powerful modulators of synaptic transmission that act on presynaptic cannabinoid receptors. Cannabinoid receptor type 1 (CB1) is the dominant receptor in the CNS, and is present in many brain regions, including sensory cortex. To investigate the potential role of CB1 receptors in cortical development, we examined the developmental expression of CB1 in rodent primary somatosensory (barrel) cortex, using immunohistochemistry with a CB1-specific antibody. We found that before postnatal day (P) 6, CB1 receptor staining was present exclusively in the cortical white matter, and that CB1 staining appeared in the gray matter between P6 and P20 in a specific laminar pattern. CB1 staining was confined to axons, and was most prominent in cortical layers 2/3, 5a, and 6. CB1 null (-/-) mice showed altered anatomical barrel maps in layer 4, with enlarged inter-barrel septa, but normal barrel size. These results indicate that CB1 receptors are present in early postnatal development and influence development of sensory maps.
Figures



Similar articles
-
Endocannabinoid signaling is required for development and critical period plasticity of the whisker map in somatosensory cortex.Neuron. 2009 Nov 25;64(4):537-49. doi: 10.1016/j.neuron.2009.10.005. Neuron. 2009. PMID: 19945395 Free PMC article.
-
Cell type specific impact of cannabinoid receptor signaling in somatosensory barrel map formation in mice.J Comp Neurol. 2020 Jan 1;528(1):3-13. doi: 10.1002/cne.24733. Epub 2019 Jul 1. J Comp Neurol. 2020. PMID: 31226222
-
Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types.J Neurosci. 2005 Jul 20;25(29):6845-56. doi: 10.1523/JNEUROSCI.0442-05.2005. J Neurosci. 2005. PMID: 16033894 Free PMC article.
-
Developmental and visual input-dependent regulation of the CB1 cannabinoid receptor in the mouse visual cortex.PLoS One. 2013;8(1):e53082. doi: 10.1371/journal.pone.0053082. Epub 2013 Jan 8. PLoS One. 2013. PMID: 23308141 Free PMC article.
-
Redistribution of CB1 cannabinoid receptors during evolution of cholinergic basal forebrain territories and their cortical projection areas: a comparison between the gray mouse lemur (Microcebus murinus, primates) and rat.Neuroscience. 2005;135(2):595-609. doi: 10.1016/j.neuroscience.2005.06.043. Neuroscience. 2005. PMID: 16129564
Cited by
-
Cannabinoid-dependent potentiation of inhibition at eye opening in mouse V1.Front Cell Neurosci. 2014 Feb 19;8:46. doi: 10.3389/fncel.2014.00046. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24600349 Free PMC article.
-
Endocannabinoid signaling is required for development and critical period plasticity of the whisker map in somatosensory cortex.Neuron. 2009 Nov 25;64(4):537-49. doi: 10.1016/j.neuron.2009.10.005. Neuron. 2009. PMID: 19945395 Free PMC article.
-
The localization and physiological effects of cannabinoid receptor 1 in the brain stem auditory system of the chick.Neuroscience. 2011 Oct 27;194:150-9. doi: 10.1016/j.neuroscience.2011.05.061. Epub 2011 Jun 7. Neuroscience. 2011. PMID: 21703331 Free PMC article.
-
Spatial organization of neuron-astrocyte interactions in the somatosensory cortex.Cereb Cortex. 2023 Apr 4;33(8):4498-4511. doi: 10.1093/cercor/bhac357. Cereb Cortex. 2023. PMID: 36124663 Free PMC article.
-
STDP in the Developing Sensory Neocortex.Front Synaptic Neurosci. 2010 Jun 9;2:9. doi: 10.3389/fnsyn.2010.00009. eCollection 2010. Front Synaptic Neurosci. 2010. PMID: 21423495 Free PMC article.
References
-
- Berrendero F, Garcia-Gil L, Hernandez ML, Romero J, Cebeira M, de Miguel R, Ramos JA, Fernandez-Ruiz JJ. Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development. 1998;125:3179–3188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

