ZO-1 is required for protein kinase C gamma-driven disassembly of connexin 43
- PMID: 17210245
- PMCID: PMC2698429
- DOI: 10.1016/j.cellsig.2006.11.007
ZO-1 is required for protein kinase C gamma-driven disassembly of connexin 43
Abstract
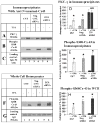
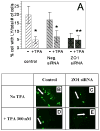
We have previously reported that protein kinase C gamma (PKC-gamma) is activated by phorbol-12-myristate-13-acetate (TPA) and that this causes PKC-gamma translocation to membranes and phosphorylation of the gap junction protein, connexin 43 (Cx43). This phosphorylation, on S368 of Cx43, causes disassembly of Cx43 out of cell junctional plaques resulting in the inhibition of dye transfer. The purpose of this study is to identify the specific role of zonula occludens protein-1 (ZO-1), a tight junction protein with recently established effects on gap junctions, in this PKC-gamma-driven Cx43 disassembly. For this purpose, ZO-1 levels in lens epithelial cells in culture were decreased by up to 70% using specific siRNA. The down-regulation of ZO-1 caused a stable interaction of PKC-gamma with Cx43 even without normal enzyme activation by TPA. However, after TPA activation of the PKC-gamma, the Cx43 did not disassemble out of plaques even though the PKC-gamma enzyme was activated and the Cx43 was phosphorylated on S368. Confocal microscopy demonstrated that the siRNA treatment caused a loss of ZO-1 from borders of large junctional Cx43 cell-to-cell plaques and resulted in the accumulation of Cx43 aggregates inside of cells. Loss of the specific "plaquetosome" arrangement of large Cx43 plaques surrounded by ZO-1 was accompanied by a complete loss of functional dye transfer. These results suggest that ZO-1 is required for Cx43 control, both for dye transfer, and, for the PKC-gamma-driven disassembly response.
Figures



References
-
- Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Physiol Rev. 2003;83:1359–1400. - PubMed
-
- Solan JL, Lampe PD. Biochim Biophys Acta. 2005;1711:154–163. - PubMed
-
- Moreno AP, Saez JC, Fishman GI, Spray DC. Circ Res. 1994;74:1050–1057. - PubMed
-
- Bao X, Reuss L, Altenberg GA. J Biol Chem. 2004;279:20058–20066. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

