Protein kinase C-delta regulates migration and proliferation of vascular smooth muscle cells through the extracellular signal-regulated kinase 1/2
- PMID: 17210402
- PMCID: PMC1829412
- DOI: 10.1016/j.jvs.2006.09.053
Protein kinase C-delta regulates migration and proliferation of vascular smooth muscle cells through the extracellular signal-regulated kinase 1/2
Abstract
Background: Smooth muscle cell (SMC) migration and proliferation are early and crucial events in the pathogenesis of intimal hyperplasia, the primary cause of restenosis after vascular intervention. We tested the hypothesis that protein kinase C-delta (PKCdelta), a ubiquitously expressed intracellular protein kinase, regulates vascular SMC proliferation and migration.
Methods: Exogenous PKCdelta was expressed in cultured SMCs via stable transfection or adenovirus-mediated gene transfer. Conversely, endogenous PKCdelta was inhibited by means of targeted gene deletion (gene knock-out). Cell proliferation and migration were determined by (3)H-thymidine incorporation and 24-well transwell assay, respectively.
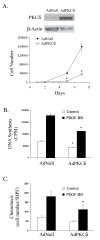
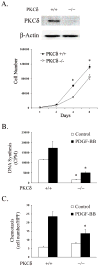
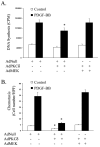
Results: We isolated and examined three A10 SMC lines in which PKCdelta was stably transfected. Compared with cells that were transfected with an empty vector, cells transfected with PKCdelta exhibited reduced ability to proliferate. Moreover, PKCdelta transfection inhibited SMC migration toward platelet-derived growth factor-BB. Similar inhibitory effects on proliferation and migration were also observed when PKCdelta was introduced into primary aortic SMCs via an adenoviral vector. Interestingly, SMCs isolated from PKCdelta knockout mice also displayed decreased chemotaxis and proliferation compared with PKCdelta(+/+) littermates, suggesting a complex yet critical role for PKCdelta. We studied the mitogen-activated protein kinase extracellular signal-regulated kinases (ERK) 1/2 as a possible signaling pathway for PKCdelta's inhibitory effect. PKCdelta overexpression diminished ERK1/2 activity. Molecular restoration of ERK activation reversed the inhibitory effect of PKCdelta on SMC proliferation and migration.
Conclusions: We demonstrate that although normal migration and proliferation is lessened in SMCs deficient in PKCdelta, its prolonged activation also diminishes those behaviors. This suggests a dual, critical role for PKCdelta in SMC proliferation and migration, and thus intimal hyperplasia and restenosis.
Figures


Similar articles
-
Protein kinase C delta activated adhesion regulates vascular smooth muscle cell migration.J Surg Res. 2007 Jul;141(1):91-6. doi: 10.1016/j.jss.2007.02.025. J Surg Res. 2007. PMID: 17574042
-
Mechanical stress-activated PKCdelta regulates smooth muscle cell migration.FASEB J. 2003 Nov;17(14):2106-8. doi: 10.1096/fj.03-0150fje. Epub 2003 Sep 4. FASEB J. 2003. PMID: 12958154
-
Histone deacetylase 4 controls neointimal hyperplasia via stimulating proliferation and migration of vascular smooth muscle cells.Hypertension. 2014 Feb;63(2):397-403. doi: 10.1161/HYPERTENSIONAHA.113.01843. Epub 2013 Oct 28. Hypertension. 2014. PMID: 24166750
-
Crosstalk between protein kinase A and growth factor receptor signaling pathways in arterial smooth muscle.Cell Signal. 1999 Jul;11(7):465-77. doi: 10.1016/s0898-6568(99)00020-0. Cell Signal. 1999. PMID: 10405757 Review.
-
Vascular smooth muscle cell migration: current research and clinical implications.Vasc Endovascular Surg. 2004 Jan-Feb;38(1):11-23. doi: 10.1177/153857440403800102. Vasc Endovascular Surg. 2004. PMID: 14760473 Review.
Cited by
-
Receptor-mediated vascular smooth muscle migration induced by LPA involves p38 mitogen-activated protein kinase pathway activation.Int J Mol Sci. 2009 Jul 13;10(7):3194-3208. doi: 10.3390/ijms10073194. Int J Mol Sci. 2009. PMID: 19742132 Free PMC article.
-
Formation of ternary complex of human biliverdin reductase-protein kinase Cδ-ERK2 protein is essential for ERK2-mediated activation of Elk1 protein, nuclear factor-κB, and inducible nitric-oxidase synthase (iNOS).J Biol Chem. 2012 Jan 6;287(2):1066-79. doi: 10.1074/jbc.M111.279612. Epub 2011 Nov 7. J Biol Chem. 2012. PMID: 22065579 Free PMC article.
-
Calcium Signaling Dynamics in Vascular Cells and Their Dysregulation in Vascular Disease.Biomolecules. 2025 Jun 18;15(6):892. doi: 10.3390/biom15060892. Biomolecules. 2025. PMID: 40563532 Free PMC article. Review.
-
ERK1/2 promotes cigarette smoke-induced rat pulmonary artery smooth muscle cells proliferation and pulmonary vascular remodeling via up-regulating cycline1 expression.J Huazhong Univ Sci Technolog Med Sci. 2013 Jun;33(3):315-322. doi: 10.1007/s11596-013-1117-8. Epub 2013 Jun 17. J Huazhong Univ Sci Technolog Med Sci. 2013. PMID: 23771653
-
PKCδ regulates the vascular biology in diabetic atherosclerosis.Cell Commun Signal. 2023 Nov 16;21(1):330. doi: 10.1186/s12964-023-01361-4. Cell Commun Signal. 2023. PMID: 37974282 Free PMC article. Review.
References
-
- Itoh H, Yamamura S, Ware JA, Zhuang S, Mii S, Liu B, et al. Differential effects of protein kinase C on human vascular smooth muscle cell proliferation and migration. Am J Physiol Heart Circ Physiol. 2001;281:H359–370. - PubMed
-
- Haller H, Lindschau C, Quass P, Distler A, Luft FC. Differentiation of Vascular Smooth Muscle Cells and the Regulation of Protein Kinase C-{alpha} Circ Res. 1995;76:21–29. - PubMed
-
- Acs P, Wang QJ, Bogi K, Marquez AM, Lorenzo PS, Biro T, et al. Both the Catalytic and Regulatory Domains of Protein Kinase C Chimeras Modulate the Proliferative Properties of NIH 3T3 Cells. J Biol Chem. 1997;272:28793–28799. - PubMed
-
- Harrington EO, Loffler J, Nelson PR, Kent KC, Simons M, Ware JA. Enhancement of Migration by Protein Kinase Calpha and Inhibition of Proliferation and Cell Cycle Progression by Protein Kinase Cdelta in Capillary Endothelial Cells. J Biol Chem. 1997;272:7390–7397. - PubMed
-
- Page K, Li J, Zhou L, Iasvoyskaia S, Corbit KC, Soh J-W, et al. Regulation of Airway Epithelial Cell NF-{kappa}B-Dependent Gene Expression by Protein Kinase C{delta} J Immunol. 2003;170:5681–5689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

