Distinct structural features of caprin-1 mediate its interaction with G3BP-1 and its induction of phosphorylation of eukaryotic translation initiation factor 2alpha, entry to cytoplasmic stress granules, and selective interaction with a subset of mRNAs
- PMID: 17210633
- PMCID: PMC1820512
- DOI: 10.1128/MCB.02300-06
Distinct structural features of caprin-1 mediate its interaction with G3BP-1 and its induction of phosphorylation of eukaryotic translation initiation factor 2alpha, entry to cytoplasmic stress granules, and selective interaction with a subset of mRNAs
Abstract
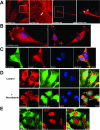
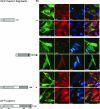
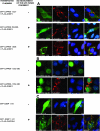
Caprin-1 is a ubiquitously expressed, well-conserved cytoplasmic phosphoprotein that is needed for normal progression through the G(1)-S phase of the cell cycle and occurs in postsynaptic granules in dendrites of neurons. We demonstrate that Caprin-1 colocalizes with RasGAP SH3 domain binding protein-1 (G3BP-1) in cytoplasmic RNA granules associated with microtubules and concentrated in the leading and trailing edge of migrating cells. Caprin-1 exhibits a highly conserved motif, F(M/I/L)Q(D/E)Sx(I/L)D that binds to the NTF-2-like domain of G3BP-1. The carboxy-terminal region of Caprin-1 selectively bound mRNA for c-Myc or cyclin D2, this binding being diminished by mutation of the three RGG motifs and abolished by deletion of the RGG-rich region. Overexpression of Caprin-1 induced phosphorylation of eukaryotic translation initiation factor 2alpha (eIF-2alpha) through a mechanism that depended on its ability to bind mRNA, resulting in global inhibition of protein synthesis. However, cells lacking Caprin-1 exhibited no changes in global rates of protein synthesis, suggesting that physiologically, the effects of Caprin-1 on translation were limited to restricted subsets of mRNAs. Overexpression of Caprin-1 induced the formation of cytoplasmic stress granules (SG). Its ability to bind RNA was required to induce SG formation but not necessarily its ability to enter SG. The ability of Caprin-1 or G3BP-1 to induce SG formation or enter them did not depend on their association with each other. The Caprin-1/G3BP-1 complex is likely to regulate the transport and translation of mRNAs of proteins involved with synaptic plasticity in neurons and cellular proliferation and migration in multiple cell types.
Figures







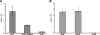
References
-
- Angenstein, F., A. M. Evans, S. C. Ling, R. E. Settlage, S. Ficarro, F. A. Carrero-Martinez, J. Shabanowitz, D. F. Hunt, and W. T. Greenough. 2005. Proteomic characterization of messenger ribonucleoprotein complexes bound to nontranslated or translated poly(A) mRNAs in the rat cerebral cortex. J. Biol. Chem. 280:6496-6503. - PubMed
-
- Angenstein, F., A. M. Evans, R. E. Settlage, S. T. Moran, S. C. Ling, A. Y. Klintsova, J. Shabanowitz, D. F. Hunt, and W. T. Greenough. 2002. A receptor for activated C kinase is part of messenger ribonucleoprotein complexes associated with polyA-mRNAs in neurons. J. Neurosci. 22:8827-8837. - PMC - PubMed
-
- Atlas, R., L. Behar, E. Elliott, and I. Ginzburg. 2004. The insulin-like growth factor mRNA binding-protein IMP-1 and the Ras-regulatory protein G3BP associate with tau mRNA and HuD protein in differentiated P19 neuronal cells. J. Neurochem. 89:613-626. - PubMed
-
- Ben-Asouli, Y., Y. Banai, Y. Pel-Or, A. Shir, and R. Kaempfer. 2002. Human interferon-gamma mRNA autoregulates its translation through a pseudoknot that activates the interferon-inducible protein kinase PKR. Cell 108:221-232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
