Neuregulin-induced ErbB3 downregulation is mediated by a protein stability cascade involving the E3 ubiquitin ligase Nrdp1
- PMID: 17210635
- PMCID: PMC1820496
- DOI: 10.1128/MCB.01245-06
Neuregulin-induced ErbB3 downregulation is mediated by a protein stability cascade involving the E3 ubiquitin ligase Nrdp1
Abstract
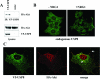
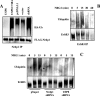
The molecular mechanisms underlying epidermal growth factor (EGF) receptor tyrosine kinase down-regulation in response to growth factor binding are coming into focus and involve cbl-mediated receptor ubiquitination followed by lysosomal degradation. However, mechanisms underlying the ligand-stimulated degradation of the related receptor tyrosine kinases of the ErbB family do not involve cbl and remain unexplored. Previous studies have demonstrated that the E3 ubiquitin ligase Nrdp1 contributes to the maintenance of steady-state ErbB3 levels by mediating its growth factor-independent degradation. Here we demonstrate that treatment of cells with the ErbB3 ligand neuregulin-1 (NRG1) stabilizes the deubiquitinating enzyme USP8, which in turn stabilizes Nrdp1. The catalytic activity of USP8 is required for NRG1-induced Nrdp1 stabilization. We provide evidence that Akt-mediated phosphorylation of USP8 threonine residue T907 contributes to USP8 stability. Finally, we demonstrate that Nrdp1 or USP8 knockdown suppresses NRG1-induced ErbB3 ubiquitination and degradation in MCF7 breast cancer cells. We conclude that an NRG1-induced protein stability cascade involving USP8 and Nrdp1 mediates the down-regulation of ErbB3. Our observations raise the possibility that the ligand-induced augmentation of pathways involved in the maintenance of basal levels of receptor tyrosine kinases can contribute to ligand-stimulated down-regulation.
Figures

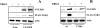






Similar articles
-
Stabilization of the E3 ubiquitin ligase Nrdp1 by the deubiquitinating enzyme USP8.Mol Cell Biol. 2004 Sep;24(17):7748-57. doi: 10.1128/MCB.24.17.7748-7757.2004. Mol Cell Biol. 2004. PMID: 15314180 Free PMC article.
-
Loss of Nrdp1 enhances ErbB2/ErbB3-dependent breast tumor cell growth.Cancer Res. 2006 Dec 1;66(23):11279-86. doi: 10.1158/0008-5472.CAN-06-2319. Cancer Res. 2006. PMID: 17145873
-
An RBCC protein implicated in maintenance of steady-state neuregulin receptor levels.Proc Natl Acad Sci U S A. 2002 Mar 5;99(5):2866-71. doi: 10.1073/pnas.052709799. Epub 2002 Feb 26. Proc Natl Acad Sci U S A. 2002. PMID: 11867753 Free PMC article.
-
The role of ErbB3 and its binding partners in breast cancer progression and resistance to hormone and tyrosine kinase directed therapies.J Mammary Gland Biol Neoplasia. 2008 Jun;13(2):225-33. doi: 10.1007/s10911-008-9077-5. Epub 2008 Apr 19. J Mammary Gland Biol Neoplasia. 2008. PMID: 18425425 Free PMC article. Review.
-
The neuregulin-I/ErbB signaling system in development and disease.Adv Anat Embryol Cell Biol. 2007;190:1-65. Adv Anat Embryol Cell Biol. 2007. PMID: 17432114 Review.
Cited by
-
Inhibition of protein synthesis alters protein degradation through activation of protein kinase B (AKT).J Biol Chem. 2013 Aug 16;288(33):23875-83. doi: 10.1074/jbc.M112.445148. Epub 2013 Jul 10. J Biol Chem. 2013. PMID: 23843462 Free PMC article.
-
Functional genomic characterization of a synthetic anti-HER3 antibody reveals a role for ubiquitination by RNF41 in the anti-proliferative response.J Biol Chem. 2019 Jan 25;294(4):1396-1409. doi: 10.1074/jbc.RA118.004420. Epub 2018 Dec 6. J Biol Chem. 2019. PMID: 30523157 Free PMC article.
-
Oligomerization of the Nrdp1 E3 ubiquitin ligase is necessary for efficient autoubiquitination but not ErbB3 ubiquitination.J Biol Chem. 2014 Mar 21;289(12):8570-8. doi: 10.1074/jbc.M113.527036. Epub 2014 Feb 11. J Biol Chem. 2014. PMID: 24519943 Free PMC article.
-
Endocytosis of receptor tyrosine kinases.Cold Spring Harb Perspect Biol. 2013 May 1;5(5):a017459. doi: 10.1101/cshperspect.a017459. Cold Spring Harb Perspect Biol. 2013. PMID: 23637288 Free PMC article. Review.
-
Anti-HER3 domain 1 and 3 antibodies reduce tumor growth by hindering HER2/HER3 dimerization and AKT-induced MDM2, XIAP, and FoxO1 phosphorylation.Neoplasia. 2013 Mar;15(3):335-47. doi: 10.1593/neo.121960. Neoplasia. 2013. PMID: 23479511 Free PMC article.
References
-
- Adam, L., R. Vadlamudi, S. B. Kondapaka, J. Chernoff, J. Mendelsohn, and R. Kumar. 1998. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J. Biol. Chem. 273:28238-28246. - PubMed
-
- Alimandi, M., A. Romano, M. C. Curia, R. Muraro, P. Fedi, S. A. Aaronson, P. P. Di Fiore, and M. H. Kraus. 1995. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene 10:1813-1821. - PubMed
-
- Alroy, I., and Y. Yarden. 1997. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 410:83-86. - PubMed
-
- Baulida, J., M. H. Kraus, M. Alimandi, P. P. Di Fiore, and G. Carpenter. 1996. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J. Biol. Chem. 271:5251-5257. - PubMed
-
- Baulida, J., and G. Carpenter. 1997. Heregulin degradation in the absence of rapid receptor-mediated internalization. Exp. Cell Res. 232:167-172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
