Cooperation and conflict in microbial biofilms
- PMID: 17210916
- PMCID: PMC1783407
- DOI: 10.1073/pnas.0607651104
Cooperation and conflict in microbial biofilms
Abstract
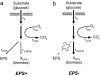
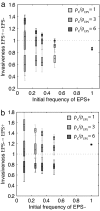
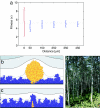
Biofilms, in which cells attach to surfaces and secrete slime (polymeric substances), are central to microbial life. Biofilms are often thought to require high levels of cooperation because extracellular polymeric substances are a shared resource produced by one cell that can be used by others. Here we examine this hypothesis by using a detailed individual-based simulation of a biofilm to investigate the outcome of evolutionary competitions between strains that differ in their level of polymer production. Our model includes a biochemical description of the carbon fluxes for growth and polymer production, and it explicitly calculates diffusion-reaction effects and the resulting solute gradients in the biofilm. An emergent property of these simple but realistic mechanistic assumptions is a strong evolutionary advantage to extracellular polymer production. Polymer secretion is altruistic to cells above a focal cell: it pushes later generations in their lineage up and out into better oxygen conditions, but it harms others; polymer production suffocates neighboring nonpolymer producers. This property, analogous to vertical growth in plants, suggests that polymer secretion provides a strong competitive advantage to cell lineages within mixed-genotype biofilms: global cooperation is not required. Our model fundamentally changes how biofilms are expected to respond to changing social conditions; the presence of multiple strains in a biofilm should promote rather than inhibit polymer secretion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappinscott HM. Annu Rev Microbiol. 1995;49:711–745. - PubMed
-
- Kolter R, Greenberg EP. Nature. 2006;441:300–302. - PubMed
-
- Fux CA, Costerton JW, Stewart PS, Stoodley P. Trends Microbiol. 2005;13:34–40. - PubMed
-
- Heijnen JJ, van Loosdrecht MCM, Mulder R, Weltevrede R, Mulder A. Wat Sci Technol. 1993;27:253–261.
-
- Kreft JU. Microbiology. 2004;150:2751–2760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

