A structural rationale for stalling of a replicative DNA polymerase at the most common oxidative thymine lesion, thymine glycol
- PMID: 17210917
- PMCID: PMC1783396
- DOI: 10.1073/pnas.0606648104
A structural rationale for stalling of a replicative DNA polymerase at the most common oxidative thymine lesion, thymine glycol
Abstract
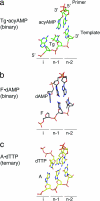
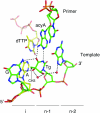
Thymine glycol (Tg) is a common product of oxidation and ionizing radiation, including that used for cancer treatment. Although Tg is a poor mutagenic lesion, it has been shown to present a strong block to both repair and replicative DNA polymerases. The 2.65-A crystal structure of a binary complex of the replicative RB69 DNA polymerase with DNA shows that the templating Tg is intrahelical and forms a regular Watson-Crick base pair with the incorporated A. The C5 methyl group protrudes axially from the ring of the damaged pyrimidine and hinders stacking of the adjacent 5' template guanine. The position of the displaced 5' template guanine is such that the next incoming nucleotide cannot be incorporated into the growing primer strand, and it explains why primer extension past the lesion is prohibited even though DNA polymerases can readily incorporate an A across from the Tg lesion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
A crystallographic study of the role of sequence context in thymine glycol bypass by a replicative DNA polymerase serendipitously sheds light on the exonuclease complex.J Mol Biol. 2011 Sep 9;412(1):22-34. doi: 10.1016/j.jmb.2011.07.007. Epub 2011 Jul 18. J Mol Biol. 2011. PMID: 21781974 Free PMC article.
-
Crystal structure of a replicative DNA polymerase bound to the oxidized guanine lesion guanidinohydantoin.Biochemistry. 2010 Mar 23;49(11):2502-9. doi: 10.1021/bi902195p. Biochemistry. 2010. PMID: 20166752 Free PMC article.
-
Structural basis of error-prone replication and stalling at a thymine base by human DNA polymerase iota.EMBO J. 2009 Jun 3;28(11):1644-54. doi: 10.1038/emboj.2009.122. EMBO J. 2009. PMID: 19440206 Free PMC article.
-
Thymidine glycol: the effect on DNA molecular structure and enzymatic processing.Biochimie. 2013 Feb;95(2):134-47. doi: 10.1016/j.biochi.2012.09.008. Epub 2012 Sep 20. Biochimie. 2013. PMID: 23000318 Review.
-
RB69 DNA polymerase structure, kinetics, and fidelity.Biochemistry. 2014 May 6;53(17):2752-67. doi: 10.1021/bi4014215. Epub 2014 Apr 23. Biochemistry. 2014. PMID: 24720884 Free PMC article. Review.
Cited by
-
Kinetics of mismatch formation opposite lesions by the replicative DNA polymerase from bacteriophage RB69.Biochemistry. 2010 Mar 23;49(11):2317-25. doi: 10.1021/bi901488d. Biochemistry. 2010. PMID: 20166748 Free PMC article.
-
The RECQL4 protein, deficient in Rothmund-Thomson syndrome is active on telomeric D-loops containing DNA metabolism blocking lesions.DNA Repair (Amst). 2013 Jul;12(7):518-28. doi: 10.1016/j.dnarep.2013.04.005. Epub 2013 May 15. DNA Repair (Amst). 2013. PMID: 23683351 Free PMC article.
-
Trimming of damaged 3' overhangs of DNA double-strand breaks by the Metnase and Artemis endonucleases.DNA Repair (Amst). 2013 Jun 1;12(6):422-32. doi: 10.1016/j.dnarep.2013.03.005. Epub 2013 Apr 18. DNA Repair (Amst). 2013. PMID: 23602515 Free PMC article.
-
Substitution of Ala for Tyr567 in RB69 DNA polymerase allows dAMP and dGMP to be inserted opposite Guanidinohydantoin.Biochemistry. 2010 Oct 5;49(39):8554-63. doi: 10.1021/bi100913v. Epub 2010 Sep 9. Biochemistry. 2010. PMID: 20795733 Free PMC article.
-
A role for DNA polymerase θ in promoting replication through oxidative DNA lesion, thymine glycol, in human cells.J Biol Chem. 2014 May 9;289(19):13177-85. doi: 10.1074/jbc.M114.556977. Epub 2014 Mar 19. J Biol Chem. 2014. PMID: 24648516 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

