Evaluation of the cancer chemopreventive efficacy of rice bran in genetic mouse models of breast, prostate and intestinal carcinogenesis
- PMID: 17211473
- PMCID: PMC2359993
- DOI: 10.1038/sj.bjc.6603539
Evaluation of the cancer chemopreventive efficacy of rice bran in genetic mouse models of breast, prostate and intestinal carcinogenesis
Abstract
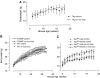
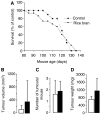
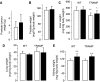
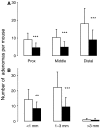
Brown rice is a staple dietary constituent in Asia, whereas rice consumed in the Western world is generally white, obtained from brown rice by removal of the bran. We tested the hypothesis that rice bran interferes with development of tumours in TAg, TRansgenic Adenocarcinoma of the Mouse Prostate (TRAMP) or Apc(Min) mice, genetic models of mammary, prostate and intestinal carcinogenesis, respectively. Mice received rice bran (30%) in AIN-93G diet throughout their post-weaning lifespan. In TAg and TRAMP mice, rice bran did not affect carcinoma development. In TRAMP or wild-type C57Bl6/J mice, dietary rice bran increased kidney weight by 18 and 20%, respectively. Consumption of rice bran reduced numbers of intestinal adenomas in Apc(Min) mice by 51% (P<0.01), compared to mice on control diet. In parallel, dietary rice bran decreased intestinal haemorrhage in these mice, as reflected by increased haematocrit. At 10% in the diet, rice bran did not significantly retard Apc(Min) adenoma development. Likewise, low-fibre rice bran (30% in the diet) did not affect intestinal carcinogenesis, suggesting that the fibrous constituents of the bran mediate chemopreventive efficacy. The results suggest that rice bran might be beneficially evaluated as a putative chemopreventive intervention in humans with intestinal polyps.
Figures

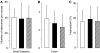
References
-
- Aoe S, Oda T, Tojima T, Tanaka M, Tatsumi K, Mizutani T (1993) Effects of rice bran hemicellulose on 1,2-dimethylhydrazine-induced intestinal carcinogenesis in Fischer 344 rats. Nutr Cancer 20: 41–49 - PubMed
-
- Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, Snover DC, Church TR, Allen JI, Beach M, Beck GJ, Bond JH, Byers T, Greenberg ER, Mandel JS, Marcon N, Mott LA, Pearson L, Saibil F, van Stolk RU (2003) A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 348: 891–899 - PubMed
-
- Boolbol SK, Dannenberg AJ, Chadburn A, Martucci C, Guo XJ, Ramonetti JT, Abreu Goris M, Newmark HL, Lipkin ML, De Cosse JJ, Bertagnolli MM (1996) Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res 56: 2556–2560 - PubMed
-
- Dyson N, Buchkovich K, Whyte P, Harlow E (1989) The cellular 107K protein that binds to adenovirus E1A also associates with the large T antigens of SV40 and JC virus. Cell 58: 249–255 - PubMed
-
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90: 1371–1388 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

