DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents
- PMID: 17211479
- PMCID: PMC2359994
- DOI: 10.1038/sj.bjc.6603515
DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents
Abstract
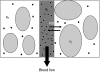
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is now frequently used in early clinical trial assessment of antiangiogenic and vascular disrupting compounds. Evidence of drug efficacy and dose-dependent response has been demonstrated with some angiogenesis inhibitors. This review highlights the critical issues that influence T(1)-weighted DCE-MRI data acquisition and analysis, identifies important areas for future development and reviews the clinical trial findings to date.
Figures
References
-
- Baluk P, Hashizume H, McDonald DM (2005) Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev 15: 102–111 - PubMed
-
- Buonaccorsi GA, Roberts C, Cheung S, Watson Y, O'Connor JP, Davies K, Jackson A, Jayson GC, Parker GJ (2006) Comparison of the performance of tracer kinetic model-driven registration for dynamic contrast enhanced MRI using different models of contrast enhancement. Acad Radiol 13: 1112–1123 - PubMed
-
- Conrad C, Friedman H, Reardon D, Provenzale J, Jackson E, Serajuddin H, Laurent D, Chen B, Yung WKA (2004) A phase I/II trial of single-agent PTK 787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in patients with recurrent glioblastoma multiforme (GBM). J Clin Oncol (Meetings Abstracts) 22(14S): 1512
-
- Dowlati A, Robertson K, Cooney M, Petros WP, Stratford M, Jesberger J, Rafie N, Overmoyer B, Makkar V, Stambler B, Taylor A, Waas J, Lewin JS, McCrae KR, Remick SC (2002) A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin a-4 phosphate on a single-dose intravenous schedule in patients with advanced cancer. Cancer Res 62: 3408–3416 - PubMed
-
- Drevs J, Medinger M, Mross K, Zirrgiebel U, Strecker R, Unger C, Puchalski TA, Fernandes N, Roberston J, Siegert P (2005) Phase I clinical evaluation of AZD2171, a highly potent VEGF receptor tyrosine kinase inhibitor, in patients with advanced tumors. J Clin Oncol (Meetings Abstracts) 23(16S): 3002
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

