Proton shuttles and phosphatase activity in soluble epoxide hydrolase
- PMID: 17212419
- PMCID: PMC2533064
- DOI: 10.1021/ja066150c
Proton shuttles and phosphatase activity in soluble epoxide hydrolase
Abstract
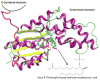
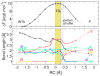
Recently, a novel metal Mg2+-dependent phosphatase activity has been discovered in the N-terminal domain of the soluble epoxide hydrolase (sEH), opening a new branch of fatty acid metabolism and providing an additional site for drug targeting. Importantly, the sEH N-terminal fold belongs to the haloacid dehalogenase (HAD) superfamily, which comprises a vast majority of phosphotransferases. Herein, we present the results of a computational study of the sEH phosphatase activity, which includes classical molecular dynamics (MD) simulations and mixed quantum mechanical/molecular mechanics (QM/MM) calculations. On the basis of experimental results, a two-step mechanism has been proposed and herein investigated: (1) phosphoenzyme intermediate formation and (2) phosphoenzyme intermediate hydrolysis. Building on our earlier work, we now provide a detailed description of the reaction mechanism for the whole catalytic cycle along with its free energy profile. The present computations suggest metaphosphate-like transition states for these phosphoryl transfers. They also reveal that the enzyme promotes water deprotonation and facilitates shuttling of protons via a metal-ligand connecting water bridge (WB). These WB-mediated proton shuttles are crucial for the activation of the solvent nucleophile and for the stabilization of the leaving group. Moreover, due to the conservation of structural features in the N-terminal catalytic site of sEH and other members of the HAD superfamily, we suggest a generalization of our findings to these other metal-dependent phosphatases.
Figures




References
-
- Morrison JF, Heyde E. Annual Reviews of Biochemistry. 1972;41:29–54. - PubMed
-
- Knowles JR. Annual Reviews of Biochemistry. 1980;49:877–919. - PubMed
-
- Jackson MD, Denu JM. Chemical Reviews. 2001;101:2313–2340. - PubMed
-
- Kennelly PJ. Chemical Reviews. 2001;101:2291–312. - PubMed
-
- Johnson LN, Lewis RJ. Chemical Reviews. 2001;101:2209–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

