Progression of renal cell carcinoma is inhibited by genistein and radiation in an orthotopic model
- PMID: 17212824
- PMCID: PMC1783858
- DOI: 10.1186/1471-2407-7-4
Progression of renal cell carcinoma is inhibited by genistein and radiation in an orthotopic model
Abstract
Background: We have previously reported the potentiation of radiotherapy by the soy isoflavone genistein for prostate cancer using prostate tumor cells in vitro and orthotopic prostate tumor models in vivo. However, when genistein was used as single therapy in animal models, it promoted metastasis to regional para-aortic lymph nodes. To clarify whether these intriguing adverse effects of genistein are intrinsic to the orthotopic prostate tumor model, or these results could also be recapitulated in another model, we used the orthotopic metastatic KCI-18 renal cell carcinoma (RCC) model established in our laboratory.
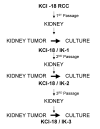
Methods: The KCI-18 RCC cell line was generated from a patient with papillary renal cell carcinoma. Following orthotopic renal implantation of KCI-18 RCC cells and serial in vivo kidney passages in nude mice, we have established a reliable and predictable metastatic RCC tumor model. Mice bearing established kidney tumors were treated with genistein combined with kidney tumor irradiation. The effect of the therapy was assessed on the primary tumor and metastases to various organs.
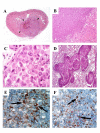
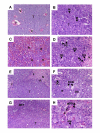
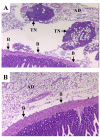
Results: In this experimental model, the karyotype and histological characteristics of the human primary tumor are preserved. Tumor cells metastasize from the primary renal tumor to the lungs, liver and mesentery mimicking the progression of RCC in humans. Treatment of established kidney tumors with genistein demonstrated a tendency to stimulate the growth of the primary kidney tumor and increase the incidence of metastasis to the mesentery lining the bowel. In contrast, when given in conjunction with kidney tumor irradiation, genistein significantly inhibited the growth and progression of established kidney tumors. These findings confirm the potentiation of radiotherapy by genistein in the orthotopic RCC model as previously shown in orthotopic models of prostate cancer.
Conclusion: Our studies in both RCC and prostate tumor models demonstrate that the combination of genistein with primary tumor irradiation is a more effective and safer therapeutic approach as the tumor growth and progression are inhibited both in the primary and metastatic sites.
Figures


Similar articles
-
Soy isoflavones enhance radiotherapy in a metastatic prostate cancer model.Int J Cancer. 2007 Jun 1;120(11):2491-8. doi: 10.1002/ijc.22548. Int J Cancer. 2007. PMID: 17304503
-
Prostate cancer treatment is enhanced by genistein in vitro and in vivo in a syngeneic orthotopic tumor model.Radiat Res. 2006 Jul;166(1 Pt 1):73-80. doi: 10.1667/RR3590.1. Radiat Res. 2006. PMID: 16808622
-
Genistein potentiates inhibition of tumor growth by radiation in a prostate cancer orthotopic model.Mol Cancer Ther. 2004 Oct;3(10):1271-9. Mol Cancer Ther. 2004. PMID: 15486194
-
Choosing the right cell line for renal cell cancer research.Mol Cancer. 2016 Dec 19;15(1):83. doi: 10.1186/s12943-016-0565-8. Mol Cancer. 2016. PMID: 27993170 Free PMC article. Review.
-
Leptomeningeal Metastases in Renal Cell Carcinoma at Initial Diagnosis: 2 Case Reports and Literature Review.Cancer Invest. 2019;37(9):501-505. doi: 10.1080/07357907.2019.1662031. Epub 2019 Oct 4. Cancer Invest. 2019. PMID: 31583922 Review.
Cited by
-
Prioritizing therapeutic targets using patient-derived xenograft models.Biochim Biophys Acta. 2015 Apr;1855(2):223-34. doi: 10.1016/j.bbcan.2015.03.002. Epub 2015 Mar 14. Biochim Biophys Acta. 2015. PMID: 25783201 Free PMC article. Review.
-
The role of isoflavones in augmenting the effects of radiotherapy.Front Oncol. 2023 Mar 1;12:800562. doi: 10.3389/fonc.2022.800562. eCollection 2022. Front Oncol. 2023. PMID: 36936272 Free PMC article. Review.
-
Investigation of the effects of treatment planning variables in small animal radiotherapy dose distributions.Med Phys. 2010 Feb;37(2):590-9. doi: 10.1118/1.3276738. Med Phys. 2010. PMID: 20229867 Free PMC article.
-
Genistein increases epidermal growth factor receptor signaling and promotes tumor progression in advanced human prostate cancer.PLoS One. 2011;6(5):e20034. doi: 10.1371/journal.pone.0020034. Epub 2011 May 16. PLoS One. 2011. PMID: 21603581 Free PMC article.
-
Genistein protects against biomarkers of delayed lung sequelae in mice surviving high-dose total body irradiation.J Radiat Res. 2008 Jul;49(4):361-72. doi: 10.1269/jrr.07121. Epub 2008 Apr 23. J Radiat Res. 2008. PMID: 18434686 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer Statistics. CA Cancer J Clin. 2006;56:106–130. - PubMed
-
- Haas GP, Hillman GG. Update on the role of immunotherapy in the management of kidney cancer. Cancer Control. 1996;3:66–71. - PubMed
-
- Mulders P, Figlin R, deKernion JB, Wiltrout R, Linehan M, Parkinson D, deWolf W, Belldegrun A. Renal cell carcinoma: recent progress and future directions. Cancer Res. 1997;57:5189–5195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

