Keratinocyte growth factor (KGF) enhances postnatal T-cell development via enhancements in proliferation and function of thymic epithelial cells
- PMID: 17213286
- PMCID: PMC1874572
- DOI: 10.1182/blood-2006-10-049767
Keratinocyte growth factor (KGF) enhances postnatal T-cell development via enhancements in proliferation and function of thymic epithelial cells
Abstract
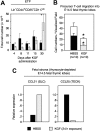
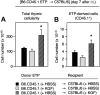
The systemic administration of keratinocyte growth factor (KGF) enhances T-cell lymphopoiesis in normal mice and mice that received a bone marrow transplant. KGF exerts protection to thymic stromal cells from cytoablative conditioning and graft-versus-host disease-induced injury. However, little is known regarding KGF's molecular and cellular mechanisms of action on thymic stromal cells. Here, we report that KGF induces in vivo a transient expansion of both mature and immature thymic epithelial cells (TECs) and promotes the differentiation of the latter type of cells. The increased TEC numbers return within 2 weeks to normal values and the microenvironment displays a normal architectural organization. Stromal changes initiate an expansion of immature thymocytes and permit regular T-cell development at an increased rate and for an extended period of time. KGF signaling in TECs activates both the p53 and NF-kappaB pathways and results in the transcription of several target genes necessary for TEC function and T-cell development, including bone morphogenetic protein 2 (BMP2), BMP4, Wnt5b, and Wnt10b. Signaling via the canonical BMP pathway is critical for the KGF effects. Taken together, these data provide new insights into the mechanism(s) of action of exogenous KGF on TEC function and thymopoiesis.
Figures

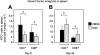

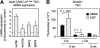

Similar articles
-
Keratinocyte growth factor preserves normal thymopoiesis and thymic microenvironment during experimental graft-versus-host disease.Blood. 2002 Jul 15;100(2):682-91. doi: 10.1182/blood.v100.2.682. Blood. 2002. PMID: 12091365
-
Short-term inhibition of p53 combined with keratinocyte growth factor improves thymic epithelial cell recovery and enhances T-cell reconstitution after murine bone marrow transplantation.Blood. 2010 Feb 4;115(5):1088-97. doi: 10.1182/blood-2009-05-223198. Epub 2009 Dec 4. Blood. 2010. PMID: 19965631 Free PMC article.
-
Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration.Blood. 2006 Mar 15;107(6):2453-60. doi: 10.1182/blood-2005-07-2831. Epub 2005 Nov 22. Blood. 2006. PMID: 16304055 Free PMC article.
-
Thymic Epithelial Cells Contribute to Thymopoiesis and T Cell Development.Front Immunol. 2020 Jan 31;10:3099. doi: 10.3389/fimmu.2019.03099. eCollection 2019. Front Immunol. 2020. PMID: 32082299 Free PMC article. Review.
-
Molecular regulatory networks of thymic epithelial cell differentiation.Differentiation. 2019 May-Jun;107:42-49. doi: 10.1016/j.diff.2019.06.002. Epub 2019 Jun 14. Differentiation. 2019. PMID: 31238242 Review.
Cited by
-
Persistent degenerative changes in thymic organ function revealed by an inducible model of organ regrowth.Aging Cell. 2012 Feb;11(1):169-77. doi: 10.1111/j.1474-9726.2011.00773.x. Epub 2011 Dec 28. Aging Cell. 2012. PMID: 22103718 Free PMC article.
-
Gene expression profile of the third pharyngeal pouch reveals role of mesenchymal MafB in embryonic thymus development.Blood. 2009 Mar 26;113(13):2976-87. doi: 10.1182/blood-2008-06-164921. Epub 2009 Jan 22. Blood. 2009. PMID: 19164599 Free PMC article.
-
Prolongevity hormone FGF21 protects against immune senescence by delaying age-related thymic involution.Proc Natl Acad Sci U S A. 2016 Jan 26;113(4):1026-31. doi: 10.1073/pnas.1514511113. Epub 2016 Jan 11. Proc Natl Acad Sci U S A. 2016. PMID: 26755598 Free PMC article.
-
Fgf21 regulates T-cell development in the neonatal and juvenile thymus.Sci Rep. 2017 Mar 23;7(1):330. doi: 10.1038/s41598-017-00349-8. Sci Rep. 2017. PMID: 28336912 Free PMC article.
-
Transplantation of Donor-Origin Mouse Embryonic Stem Cell-Derived Thymic Epithelial Progenitors Prevents the Development of Chronic Graft-versus-Host Disease in Mice.Stem Cells Transl Med. 2017 Jan;6(1):121-130. doi: 10.5966/sctm.2016-0012. Epub 2016 Aug 2. Stem Cells Transl Med. 2017. PMID: 28170174 Free PMC article.
References
-
- Mackall CL, Gress RE. Pathways of T-cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy. Immunol Rev. 1997;157:61–72. - PubMed
-
- Hakim FT, Gress RE. Reconstitution of thymic function after stem cell transplantation in humans. Curr Opin Hematol. 2002;9:490–496. - PubMed
-
- Van den Brink MR, Alpdogan O, Boyd RL. Strategies to enhance T-cell reconstitution in immunocompromised patients. Nat Rev Immunol. 2004;4:856–867. - PubMed
-
- Fry TJ, Mackall CL. Immune reconstitution following hematopoietic progenitor cell transplantation: challenges for the future. Bone Marrow Transplant. 2005;35(suppl 1):S53–S57. - PubMed
-
- Godthelp BC, van Tol MJ, Vossen JM, van Den Elsen PJ. T-cell immune reconstitution in pediatric leukemia patients after allogeneic bone marrow transplantation with T-cell-depleted or unmanipulated grafts: evaluation of overall and antigen-specific T-cell repertoires. Blood. 1999;94:4358–4369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

