In the regulation of cytochrome P450 genes, phenobarbital targets LKB1 for necessary activation of AMP-activated protein kinase
- PMID: 17213310
- PMCID: PMC1783361
- DOI: 10.1073/pnas.0610216104
In the regulation of cytochrome P450 genes, phenobarbital targets LKB1 for necessary activation of AMP-activated protein kinase
Abstract
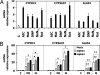
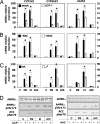
Transcriptional activation of cytochrome P450 (CYP) genes and various drug metabolizing enzymes by the prototypical inducer phenobarbital (PB) and many other drugs and chemicals is an adaptive response of the organism to exposure to xenobiotics. The response to PB is mediated by the nuclear receptor constitutive androstane receptor (CAR), whereas the chicken xenobiotic receptor (CXR) has been characterized as the PB mediator in chicken hepatocytes. Our previous results suggested an involvement of AMP-activated protein kinase (AMPK) in the molecular mechanism of PB induction. Here, we show that the mechanism of AMPK activation is related to an effect of PB-type inducers on mitochondrial function with consequent formation of reactive oxygen species (ROS) and phosphorylation of AMPK by the upstream kinase LKB1. Gain- and loss-of-function experiments demonstrate that LKB1-activated AMPK is necessary in the mechanism of drug induction and that this is an evolutionary conserved pathway for detoxification of exogenous and endogenous chemicals. The activation of LKB1 adds a proximal target to the so far elusive sequence of events by which PB and other drugs induce the transcription of multiple genes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
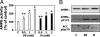


Similar articles
-
Conservation of signaling pathways of xenobiotic-sensing orphan nuclear receptors, chicken xenobiotic receptor, constitutive androstane receptor, and pregnane X receptor, from birds to humans.Mol Endocrinol. 2001 Sep;15(9):1571-85. doi: 10.1210/mend.15.9.0701. Mol Endocrinol. 2001. PMID: 11518807
-
Docosahexaenoic acid downregulates phenobarbital-induced cytochrome P450 2B1 gene expression in rat primary hepatocytes via the c-Jun NH2-terminal kinase mitogen-activated protein kinase pathway.Mol Nutr Food Res. 2009 Mar;53(3):341-8. doi: 10.1002/mnfr.200800112. Mol Nutr Food Res. 2009. PMID: 18803253
-
Transcriptional activation of cytochrome P450 CYP2C45 by drugs is mediated by the chicken xenobiotic receptor (CXR) interacting with a phenobarbital response enhancer unit.J Biol Chem. 2002 May 3;277(18):15647-53. doi: 10.1074/jbc.M109882200. Epub 2002 Feb 26. J Biol Chem. 2002. PMID: 11867618
-
Phenobarbital-induced expression of cytochrome P450 genes.Acta Biochim Pol. 2000;47(4):1093-105. Acta Biochim Pol. 2000. PMID: 11996099 Review.
-
Phenobarbital-elicited activation of nuclear receptor CAR in induction of cytochrome P450 genes.Biochem Biophys Res Commun. 2000 Oct 14;277(1):1-6. doi: 10.1006/bbrc.2000.3557. Biochem Biophys Res Commun. 2000. PMID: 11027630 Review.
Cited by
-
Activation of the AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin.Diabetes. 2009 Oct;58(10):2246-57. doi: 10.2337/db08-1512. Epub 2009 Jul 10. Diabetes. 2009. PMID: 19592618 Free PMC article.
-
AMPK in cardiovascular health and disease.Acta Pharmacol Sin. 2010 Sep;31(9):1075-84. doi: 10.1038/aps.2010.139. Epub 2010 Aug 16. Acta Pharmacol Sin. 2010. PMID: 20711221 Free PMC article. Review.
-
Pregnane X receptor and constitutive androstane receptor at the crossroads of drug metabolism and energy metabolism.Drug Metab Dispos. 2010 Dec;38(12):2091-5. doi: 10.1124/dmd.110.035568. Epub 2010 Aug 24. Drug Metab Dispos. 2010. PMID: 20736325 Free PMC article. Review.
-
Cell signaling and nuclear receptors: new opportunities for molecular pharmaceuticals in liver disease.Mol Pharm. 2008 Jan-Feb;5(1):17-34. doi: 10.1021/mp700098c. Epub 2007 Dec 27. Mol Pharm. 2008. PMID: 18159925 Free PMC article. Review.
-
Activation of CAR and PXR by Dietary, Environmental and Occupational Chemicals Alters Drug Metabolism, Intermediary Metabolism, and Cell Proliferation.Curr Pharmacogenomics Person Med. 2009 Jun 1;7(2):81-105. doi: 10.2174/187569209788654005. Curr Pharmacogenomics Person Med. 2009. PMID: 20871735 Free PMC article.
References
-
- Handschin C, Meyer UA. Pharmacol Rev. 2003;55:649–673. - PubMed
-
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, Negishi M. Mol Pharmacol. 2002;61:1–6. - PubMed
-
- Kakizaki S, Yamamoto Y, Ueda A, Moore R, Sueyoshi T, Negishi M. Biochim Biophys Acta. 2003;1619:239–242. - PubMed
-
- Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. J Biol Chem. 1999;274:6043–6046. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

