Randomized dose-ranging controlled trial of AQ-13, a candidate antimalarial, and chloroquine in healthy volunteers
- PMID: 17213921
- PMCID: PMC1764434
- DOI: 10.1371/journal.pctr.0020006
Randomized dose-ranging controlled trial of AQ-13, a candidate antimalarial, and chloroquine in healthy volunteers
Abstract
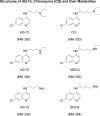
Objectives: To determine: (1) the pharmacokinetics and safety of an investigational aminoquinoline active against multidrug-resistant malaria parasites (AQ-13), including its effects on the QT interval, and (2) whether it has pharmacokinetic and safety profiles similar to chloroquine (CQ) in humans.
Design: Phase I double-blind, randomized controlled trials to compare AQ-13 and CQ in healthy volunteers. Randomizations were performed at each step after completion of the previous dose.
Setting: Tulane-Louisiana State University-Charity Hospital General Clinical Research Center in New Orleans.
Participants: 126 healthy adults 21-45 years of age.
Interventions: 10, 100, 300, 600, and 1,500 mg oral doses of CQ base in comparison with equivalent doses of AQ-13.
Outcome measures: Clinical and laboratory adverse events (AEs), pharmacokinetic parameters, and QT prolongation.
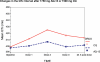
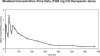
Results: No hematologic, hepatic, renal, or other organ toxicity was observed with AQ-13 or CQ at any dose tested. Headache, lightheadedness/dizziness, and gastrointestinal (GI) tract-related symptoms were the most common AEs. Although symptoms were more frequent with AQ-13, the numbers of volunteers who experienced symptoms with AQ-13 and CQ were similar (for AQ-13 and CQ, respectively: headache, 17/63 and 10/63, p = 0.2; lightheadedness/dizziness, 11/63 and 8/63, p = 0.6; GI symptoms, 14/63 and 13/63; p = 0.9). Both AQ-13 and CQ exhibited linear pharmacokinetics. However, AQ-13 was cleared more rapidly than CQ (respectively, median oral clearance 14.0-14.7 l/h versus 9.5-11.3 l/h; p < or = 0.03). QTc prolongation was greater with CQ than AQ-13 (CQ: mean increase of 28 ms; 95% confidence interval [CI], 18 to 38 ms, versus AQ-13: mean increase of 10 ms; 95% CI, 2 to 17 ms; p = 0.01). There were no arrhythmias or other cardiac AEs with either AQ-13 or CQ.
Conclusions: These studies revealed minimal differences in toxicity between AQ-13 and CQ, and similar linear pharmacokinetics.
Conflict of interest statement
Figures



References
-
- Breman JG, Alilo MS, Mills A. Conquering the intolerable burden of malaria: What's new, what's needed: A summary. Am J Trop Med Hyg. 2004;71((Suppl 2)):1–15. - PubMed
-
- World Health Organization Expert Committee on Malaria. WHO Tech Rep Ser. Vol. 892. Geneva: WHO; 2000. World Health Organization Expert Committee on Malaria (2000) pp. i–v.pp. 1–74. - PubMed
-
- Baird JK. Effectiveness of antimalarial drugs. N Engl J Med. 2005;352:1565–1577. - PubMed
-
- White NJ. The treatment of malaria. N Engl J Med. 1996;335:800–806. - PubMed
-
- Wellems TE, Plowe CV. Chloroquine-resistant malaria. J Infect Dis. 2001;184:770–776. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

