Impact of atorvastatin treatment on platelet-activating factor acetylhydrolase and 15-F(2trans)-isoprostane in hypercholesterolaemic patients
- PMID: 17214829
- PMCID: PMC2000588
- DOI: 10.1111/j.1365-2125.2006.02832.x
Impact of atorvastatin treatment on platelet-activating factor acetylhydrolase and 15-F(2trans)-isoprostane in hypercholesterolaemic patients
Abstract
Aims: Isoprostanes are the product of free radical oxidation of arachidonic acid, whose hydrolysis from phospholipids is presumably catalysed by phospholipases A(2) (PLA(2)s) such as group IIA or V PLA(2)s, or group VII PLA(2)[platelet-activating factor acetylhydrolase (PAF-AH), lipoprotein-associated phospholipase]. Atorvastatin reduces concentrations of low-density lipoprotein (LDL), with which PAF-AH is associated, and PLA(2)s' protein concentrations. We investigated the effect of atorvastatin on PLA(2)s and PAF-AH activity and the urinary excretion of 15-F(2trans)-isoprostane (15-F(2t)-IsoP, 8-iso-PGF(2alpha), iPF(2alpha)-III).
Methods: Twenty-four hypercholesterolaemic individuals naive to lipid-lowering therapy were randomized to atorvastatin 40 mg or placebo for 6 weeks. The 15-F(2t)-isoP urinary excretion (gas chromatography/mass spectrometry), PAF-AH and group IIA and V PLA(2) activities (photometry) were assessed at baseline and end-point.
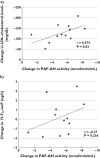
Results: At end-point, 15-F(2t)-isoP urinary excretion concentrations as well as PLA(2)s' activity were unchanged under atorvastatin (mean change 0.21 +/- 1.79 ng h(-1), 95% confidence interval -0.92, 1.35 and 0.33 +/- 0.94 nmol min(-1) ml(-1), -0.27, 0.93) and under placebo (mean change 0.69 +/- 1.69 ng h(-1), -0.52, 1.90 and 1.29 +/- 2.16 nmol min(-1) ml(-1), -0.25, 2.84). Atorvastatin treatment decreased total (P < 0.001) and LDL-cholesterol (P < 0.001) but had no effect on high-density lipoprotein. PAF-AH activity was lowered in the atorvastatin group (mean change - 5.27+/- 1.96 nmol min(-1) ml(-1), -6.51, -4.03, P < 0.001) but not in the placebo group (mean change 1.02 +/- 1.64 nmol min(-1) ml(-1), 0.15, 2.20), and the change in PAF-AH activity was correlated with that in total (P = 0.03) and LDL-cholesterol (P = 0.03).
Conclusion: Our results show a lowering effect of atorvastatin on PAF-AH activity associated with its lipid-lowering effect and exclude a key role of PAF-AH in the liberation of 15-F(2t)-isoP from phospholipids.
Figures

References
-
- Morrow JD, Roberts LJ., II The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res. 1997;36:1–21. - PubMed
-
- Cracowski JL, Devillier P, Durand T, Stanke-Labesque F, Bessard G. Vascular biology of the isoprostanes. J Vasc Res. 2001;38:93–103. - PubMed
-
- Vassalle C, Botto N, Andreassi MG, Berti S, Biagini A. Evidence for enhanced 8-isoprostane plasma levels, as index of oxidative stress in vivo, in patients with coronary artery disease. Coron Artery Dis. 2003;14:213–8. - PubMed
-
- Schwedhelm E, Bartling A, Lenzen H, Tsikas D, Maas R, Brümmer J, Gutzki FM, Berger J, Frölich JC, Böger RH. Urinary 8-iso-Prostaglandin F2α as a risk marker in patients with coronary heart disease; a matched case–control study. Circulation. 2004;109:843–8. - PubMed
-
- Devaraj S, Hirany SV, Burk RF, Jialal I. Divergence between LDL oxidative susceptibility and urinary F2-isoprostanes as measures of oxidative stress in type 2 diabetes mellitus. Clin Chem. 2001;47:1974–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

