The impact of vitamin D status on changes in bone mineral density during treatment with bisphosphonates and after discontinuation following long-term use in post-menopausal osteoporosis
- PMID: 17214897
- PMCID: PMC1781939
- DOI: 10.1186/1471-2474-8-3
The impact of vitamin D status on changes in bone mineral density during treatment with bisphosphonates and after discontinuation following long-term use in post-menopausal osteoporosis
Abstract
Background: It is still unclear whether addition of calcium/vitamin D supplements leads to an incremental benefit in patients taking bisphosphonates and whether achievement of serum level of 25 (OH) vitamin D of at least 70 nmol/L has an impact on the skeletal response to bisphosphonates. Moreover the maintenance of BMD after bisphosphonates withdrawal with the continuation of calcium/vitamin D supplements only, remains uncertain. The aims were to assess the impact of vitamin D status on changes in bone mineral density (BMD) in firstly patients with post-menopausal osteoporosis on bisphosphonates and secondly following discontinuation of bisphosphonates after long-term use.
Methods: Two patient groups were recruited. The first study population comprised of 112 women treated with a bisphosphonate. The second study population consisted of 35 women who had been on bisphosphonates for > 5 years in whom the treatment agent was discontinued. Baseline BMD, changes in BMD following treatment, duration of treatment, serum 25 (OH) vitamin D, parathyroid hormone (PTH), urine C-terminal telopeptides of type 1 collagen (CTX) were obtained on the study participants.
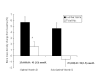
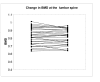
Results: In the first study group, subjects with serum vitamin D concentrations (> 70 nmol/L) had a significantly lower serum PTH level (mean [SEM] 41 2 ng/L). PTH concentrations of 41 ng/L or less was associated with a significantly higher increase in BMD at the hip following treatment with bisphosphonates compared to patients with PTH > 41 ng/L (2.5% [0.9] v/s -0.2% [0.9], P = 0.04). In the second study group, discontinuation of bisphosphonate for 15 months after long-term treatment did not result in significant bone loss at the lumbar spine and total hip, although a trend towards gradual decline in BMD at the femoral neck was observed.
Conclusion: the data suggest that optimal serum 25 (OH) vitamin D concentration may lead to further reduction in bone loss at the hip in patients on bisphosphonates. A prospective controlled trial is needed to evaluate whether the response to bisphosphonates is influenced by vitamin D status. BMD is preserved at the lumbar spine and total hip following discontinuation of bisphosphonate for a short period following long-term treatment, although a gradual loss occurs at the femoral neck.
Figures





Similar articles
-
Relationship between increased endogenous parathormone levels and bone density in postmenopausal women treated with bisphosphonates.Panminerva Med. 2012 Dec;54(4):277-82. Panminerva Med. 2012. PMID: 23123579
-
Vitamin D status, bone mass, and bone metabolism in home-dwelling postmenopausal Japanese women: Yokogoshi Study.Bone. 2008 Feb;42(2):271-7. doi: 10.1016/j.bone.2007.09.056. Epub 2007 Oct 10. Bone. 2008. PMID: 18006400
-
Secondary hyperparathyroidism due to hypovitaminosis D affects bone mineral density response to alendronate in elderly women with osteoporosis: a randomized controlled trial.J Am Geriatr Soc. 2007 May;55(5):752-7. doi: 10.1111/j.1532-5415.2007.01161.x. J Am Geriatr Soc. 2007. PMID: 17493196 Clinical Trial.
-
Significance of a decline in bone mineral density while receiving oral bisphosphonate treatment.Clin Ther. 2008 Mar;30(3):443-52. doi: 10.1016/j.clinthera.2008.03.008. Clin Ther. 2008. PMID: 18405784 Review.
-
A systematic review and meta-analysis of the effect of bisphosphonate drug holidays on bone mineral density and osteoporotic fracture risk.Osteoporos Int. 2019 Apr;30(4):705-720. doi: 10.1007/s00198-018-4791-3. Epub 2019 Jan 8. Osteoporos Int. 2019. PMID: 30623214 Free PMC article.
Cited by
-
Clinical characteristics associated with bone mineral density improvement after 1-year alendronate/vitamin d3 or calcitriol treatment: Exploratory results from a phase 3, randomized, controlled trial on postmenopausal osteoporotic women in China.Medicine (Baltimore). 2018 Aug;97(31):e11694. doi: 10.1097/MD.0000000000011694. Medicine (Baltimore). 2018. PMID: 30075569 Free PMC article. Clinical Trial.
-
Vitamin D levels and response to biphosphonates in postmenopausal women receiving glucocorticoid therapy.Osteoporos Int. 2014 Aug;25(8):2157-8. doi: 10.1007/s00198-014-2713-6. Epub 2014 May 7. Osteoporos Int. 2014. PMID: 24803328 No abstract available.
-
Oral Calcidiol Is More Effective Than Cholecalciferol Supplementation to Reach Adequate 25(OH)D Levels in Patients with Autoimmune Diseases Chronically Treated with Low Doses of Glucocorticoids: A "Real-Life" Study.J Osteoporos. 2015;2015:729451. doi: 10.1155/2015/729451. Epub 2015 Jun 1. J Osteoporos. 2015. PMID: 26124976 Free PMC article.
-
Position Statement: Vitamin D Intake to Prevent Osteoporosis and Fracture in Adults.J Bone Metab. 2022 Nov;29(4):205-215. doi: 10.11005/jbm.2022.29.4.205. Epub 2022 Nov 30. J Bone Metab. 2022. PMID: 36529863 Free PMC article.
-
Vitamin D status and response to treatment in post-menopausal osteoporosis.Osteoporos Int. 2009 Feb;20(2):239-44. doi: 10.1007/s00198-008-0650-y. Epub 2008 Jun 13. Osteoporos Int. 2009. PMID: 18551242
References
-
- Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR, Fracture Intervention Trial Fracture risk reduction with alendronate in women with osteoporosis: The Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85:4118–24. doi: 10.1210/jc.85.11.4118. - DOI - PubMed
-
- McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY, Hip Intervention Program Study Group Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–40. doi: 10.1056/NEJM200102013440503. - DOI - PubMed
-
- Porthouse J, Cockayne S, King C, Saxon L, Steele E, Aspray T, Baverstock M, Birks Y, Dumville J, Francis R, Iglesias C, Puffer S, Sutcliffe A, Watt I, Torgensen DJ. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. British Medical Journal. 2005;330:1003. doi: 10.1136/bmj.330.7498.1003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

