Classical swine fever virus Npro interacts with interferon regulatory factor 3 and induces its proteasomal degradation
- PMID: 17215286
- PMCID: PMC1866024
- DOI: 10.1128/JVI.02032-06
Classical swine fever virus Npro interacts with interferon regulatory factor 3 and induces its proteasomal degradation
Abstract
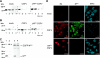
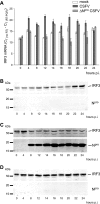
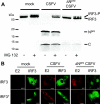
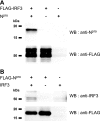
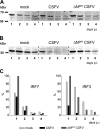
Viruses have evolved a multitude of strategies to subvert the innate immune system by interfering with components of the alpha/beta interferon (IFN-alpha/beta) induction and signaling pathway. It is well established that the pestiviruses prevent IFN-alpha/beta induction in their primary target cells, such as epitheloidal and endothelial cells, macrophages, and conventional dendritic cells, a phenotype mediated by the viral protein N(pro). Central players in the IFN-alpha/beta induction cascade are interferon regulatory factor 3 (IRF3) and IRF7. Recently, it was proposed that classical swine fever virus (CSFV), the porcine pestivirus, induced the loss of IRF3 by inhibiting the transcription of IRF3 mRNA. In the present study, we show that endogenous IRF3 and IRF3 expressed from a cytomegalovirus (CMV) promoter are depleted in the presence of CSFV by means of N(pro), while CSFV does not inhibit CMV promoter-driven protein expression. We also demonstrate that CSFV does not reduce the transcriptional activity of the IRF3 promoter and does not affect the stability of IRF3 mRNA. In fact, CSFV N(pro) induces proteasomal degradation of IRF3, as demonstrated by proteasome inhibition studies. Furthermore, N(pro) coprecipitates with IRF3, suggesting that the proteasomal degradation of IRF3 is induced by a direct or indirect interaction with N(pro). Finally, we show that N(pro) does not downregulate IRF7 expression.
Figures


References
-
- Baigent, S. J., S. Goodbourn, and J. W. McCauley. 2004. Differential activation of interferon regulatory factors-3 and -7 by non-cytopathogenic and cytopathogenic bovine viral diarrhoea virus. Vet. Immunol. Immunopathol. 100:135-144. - PubMed
-
- Balmelli, C., I. E. Vincent, H. Rau, L. Guzylack-Piriou, K. McCullough, and A. Summerfield. 2005. FcγRII-dependent sensitisation of natural interferon-producing cells for viral infection and interferon-alpha responses. Eur. J. Immunol. 35:2406-2415. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

