Arabidopsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates
- PMID: 17215350
- PMCID: PMC1783366
- DOI: 10.1073/pnas.0605423104
Arabidopsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates
Abstract
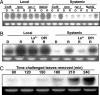
In the absence of adaptive immunity displayed by animals, plants respond locally to biotic challenge via inducible basal defense networks activated through recognition and response to conserved pathogen-associated molecular patterns. In addition, immunity can be induced in tissues remote from infection sites by systemic acquired resistance (SAR), initiated after gene-for-gene recognition between plant resistance proteins and microbial effectors. The nature of the mobile signal and remotely activated networks responsible for establishing SAR remain unclear. Salicylic acid (SA) participates in the local and systemic response, but SAR does not require long-distance translocation of SA. Here, we show that, despite the absence of pathogen-associated molecular pattern contact, systemically responding leaves rapidly activate a SAR transcriptional signature with strong similarity to local basal defense. We present several lines of evidence that suggest jasmonates are central to systemic defense, possibly acting as the initiating signal for classic SAR. Jasmonic acid (JA), but not SA, rapidly accumulates in phloem exudates of leaves challenged with an avirulent strain of Pseudomonas syringae. In systemically responding leaves, transcripts associated with jasmonate biosynthesis are up-regulated within 4 h, and JA increases transiently. SAR can be mimicked by foliar JA application and is abrogated in mutants impaired in jasmonate synthesis or response. We conclude that jasmonate signaling appears to mediate long-distance information transmission. Moreover, the systemic transcriptional response shares extraordinary overlap with local herbivory and wounding responses, indicating that jasmonates may be pivotal to an evolutionarily conserved signaling network that decodes multiple abiotic and biotic stress signals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


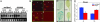

References
-
- Nurnberger T, Brunner F, Kemmerling B, Piater L. Immunol Rev. 2004;198:249–266. - PubMed
-
- Nomura K, Melotto M, He SY. Curr Opin Plant Biol. 2005;8:361–368. - PubMed
-
- Belkhadir Y, Subramaniam R, Dangl JL. Curr Opin Plant Biol. 2004;7:391–399. - PubMed
-
- Dangl JL, Jones JD. Nature. 2001;411:826–833. - PubMed
-
- Durrant WE, Dong X. Annu Rev Phytopathol. 2004;42:185–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

