Hepatocyte growth factor acts as a motogen and guidance signal for gonadotropin hormone-releasing hormone-1 neuronal migration
- PMID: 17215404
- PMCID: PMC6672060
- DOI: 10.1523/JNEUROSCI.4979-06.2007
Hepatocyte growth factor acts as a motogen and guidance signal for gonadotropin hormone-releasing hormone-1 neuronal migration
Abstract
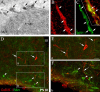
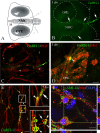
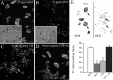
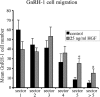
Reproduction in mammals is under the control of the hypothalamic neuropeptide gonadotropin hormone-releasing hormone-1 (GnRH-1). GnRH-1-secreting neurons originate during embryonic development in the nasal placode and migrate into the forebrain along olfactory nerves. Gradients of secreted molecules may play a role in this migratory process. In this context, hepatocyte growth factor (HGF) is a potential candidate, because it promotes cell motility in developing brain and has been shown previously to act as a motogen on immortalized GnRH-1 neurons (GN11). In this study, the role of HGF and its receptor Met during development of the GnRH-1 system was examined. GnRH-1 cells express Met during their migration and downregulate its expression once they complete this process. Tissue-type plasminogen activator (tPA), a known HGF activator, is also detected in migratory GnRH-1 neurons. Consistent with in vivo expression, HGF is present in nasal explants, and GnRH-1 neurons express Met. HGF-neutralizing antibody was applied to explants to examine the role of the endogenous growth factor. Migration of GnRH-1 cells and olfactory axon outgrowth were significantly reduced, in line with disruption of a guidance gradient. Exogenous application of HGF to explants increased the distance that GnRH-1 cells migrated, suggesting that HGF also acts as a motogen to GnRH-1 neurons. Functional experiments, performed on organotypic slice cultures, show that creation of an opposing HGF gradient inhibits GnRH-1 neuronal migration. Finally, tPA(-/-):uPA(-/-) (urokinase-type plasminogen activator(-/-)) knock-out mice exhibit strong reduction of the GnRH-1 cell population. Together, these data indicate that HGF signaling via Met receptor influences the development of GnRH-1.
Figures





Similar articles
-
Hepatocyte growth factor/scatter factor facilitates migration of GN-11 immortalized LHRH neurons.Endocrinology. 2002 Sep;143(9):3306-15. doi: 10.1210/en.2002-220146. Endocrinology. 2002. PMID: 12193542
-
Cholecystokinin modulates migration of gonadotropin-releasing hormone-1 neurons.J Neurosci. 2004 May 19;24(20):4737-48. doi: 10.1523/JNEUROSCI.0649-04.2004. J Neurosci. 2004. PMID: 15152034 Free PMC article.
-
Insulinlike growth factor-I-mediated migration and invasion of human colon carcinoma cells requires activation of c-Met and urokinase plasminogen activator receptor.Ann Surg. 2005 May;241(5):748-56; discussion 756-8. doi: 10.1097/01.sla.0000160699.59061.92. Ann Surg. 2005. PMID: 15849510 Free PMC article.
-
Factors involved in the migration of neuroendocrine hypothalamic neurons.Arch Ital Biol. 2005 Sep;143(3-4):171-8. Arch Ital Biol. 2005. PMID: 16097493 Review.
-
Gonadotropin-releasing hormone neuronal migration.Semin Reprod Med. 2007 Sep;25(5):305-12. doi: 10.1055/s-2007-984736. Semin Reprod Med. 2007. PMID: 17710726 Review.
Cited by
-
Fate mapping reveals mixed embryonic origin and unique developmental codes of mouse forebrain septal neurons.Commun Biol. 2022 Oct 27;5(1):1137. doi: 10.1038/s42003-022-04066-5. Commun Biol. 2022. PMID: 36302841 Free PMC article.
-
HGF and MET: From Brain Development to Neurological Disorders.Front Cell Dev Biol. 2021 Jun 9;9:683609. doi: 10.3389/fcell.2021.683609. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34179015 Free PMC article. Review.
-
P75 nerve growth factor receptors modulate development of GnRH neurons and olfactory ensheating cells.Front Neurosci. 2013 Dec 27;7:262. doi: 10.3389/fnins.2013.00262. eCollection 2013. Front Neurosci. 2013. PMID: 24409113 Free PMC article.
-
Dysfunctional SEMA3E signaling underlies gonadotropin-releasing hormone neuron deficiency in Kallmann syndrome.J Clin Invest. 2015 Jun;125(6):2413-28. doi: 10.1172/JCI78448. Epub 2015 May 18. J Clin Invest. 2015. PMID: 25985275 Free PMC article.
-
Growth Factors as Axon Guidance Molecules: Lessons From in vitro Studies.Front Neurosci. 2021 May 21;15:678454. doi: 10.3389/fnins.2021.678454. eCollection 2021. Front Neurosci. 2021. PMID: 34093120 Free PMC article. Review.
References
-
- Achim CL, Katyal S, Wiley CA, Shiratori M, Wang G, Oshika E, Petersen BE, Li JM, Michalopoulos GK. Expression of HGF and cMet in the developing and adult brain. Brain Res Dev Brain Res. 1997;102:299–303. - PubMed
-
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. - PubMed
-
- Bless EP, Westaway WA, Schwarting GA, Tobet SA. Effects of gamma-aminobutyric acid(A) receptor manipulation on migrating gonadotropin-releasing hormone neurons through the entire migratory route in vivo and in vitro. Endocrinology. 2000;141:1254–1262. - PubMed
-
- Calof AL, Chikaraishi DM. Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron. 1989;3:115–127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
