Structural insights into the innate immune recognition specificities of L- and H-ficolins
- PMID: 17215869
- PMCID: PMC1783469
- DOI: 10.1038/sj.emboj.7601500
Structural insights into the innate immune recognition specificities of L- and H-ficolins
Abstract
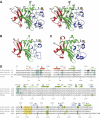
Innate immunity relies critically upon the ability of a few pattern recognition molecules to sense molecular markers on pathogens, but little is known about these interactions at the atomic level. Human L- and H-ficolins are soluble oligomeric defence proteins with lectin-like activity, assembled from collagen fibers prolonged by fibrinogen-like recognition domains. The X-ray structures of their trimeric recognition domains, alone and in complex with various ligands, have been solved to resolutions up to 1.95 and 1.7 A, respectively. Both domains have three-lobed structures with clefts separating the distal parts of the protomers. Ca(2+) ions are found at sites homologous to those described for tachylectin 5A (TL5A), an invertebrate lectin. Outer binding sites (S1) homologous to the GlcNAc-binding pocket of TL5A are present in the ficolins but show different structures and specificities. In L-ficolin, three additional binding sites (S2-S4) surround the cleft. Together, they define an unpredicted continuous recognition surface able to sense various acetylated and neutral carbohydrate markers in the context of extended polysaccharides such as 1,3-beta-D-glucan, as found on microbial or apoptotic surfaces.
Figures

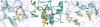


References
-
- Aoyagi Y, Adderson EE, Min JG, Matsushita M, Fujita T, Takahashi S, Okuwaki Y, Bohnsack JF (2005) Role of L-ficolin/mannose-binding lectin associated serine protease complexes in the opsonophagocytosis of type III group B streptococci. J Immunol 174: 418–425 - PubMed
-
- Bilyy RO, Antonyuk VO, Stoika RS (2004) Cytochemical study of role of α-D-mannose- and β-D-galactose-containing glycoproteins in apoptosis. J Mol Histol 35: 829–838 - PubMed
-
- Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D 54: 905–921 - PubMed
-
- Chang CI, Chelliah Y, Borek D, Mengin-Lecreulx D, Deisenhofer J (2006) Structure of tracheal cytotoxin in complex with a heterodimeric pattern-recognition receptor. Science 311: 1761–1764 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

