Highly efficient synthesis of enantiomerically enriched 2-hydroxymethylaziridines by enzymatic desymmetrization
- PMID: 17217268
- PMCID: PMC2529399
- DOI: 10.1021/ol062643a
Highly efficient synthesis of enantiomerically enriched 2-hydroxymethylaziridines by enzymatic desymmetrization
Abstract
Both enantiomers of protected and unprotected 2-hydroxymethylaziridines are efficiently and enantiospecifically synthesized by using a combination of enzymatic and synthetic methods. PPL was used for lipase-catalyzed desymmetrization of N-protected serinol. [reaction: see text].
Figures
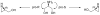
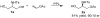




References
-
-
For reviews on the synthesis and reactions of chiral aziridines, see: Sweeney JB. Chem Soc Rev. 2002;31:247.McCoull W, Davis FA. Synthesis. 2000:1347.Tanner D. Angew Chem. 1994;106:625.Angew Chem Int Ed Engl. 1994;33:599.Müller P, Fruit C. Chem Rev. 2003;103:2905. and references therein.
-
-
- Kim SK, Jacobsen EN. Angew Chem Int Ed Engl. 2004;43:3952. - PubMed
-
- Gillespie KM, Sanders CJ. J Org Chem. 2002;67:3450. - PubMed
-
-
A few direct aziridinations of styrene have been reported: Nishikori H, Katsuki T. Tetrahedron Lett. 1996;37:9245.Li Z, Conser KR, Jacobsen EN. J Am Chem Soc. 1993;115:5326.Evans DA, Faul MM, Bilodeau MT, Anderson BA, Barnes DM. J Am Chem Soc. 1993;115:5328.
-
-
-
Asymmetirc synthesis of 2-hydroxymethylazirine has been briefly reported from chiral amino acid without isolation and characterization: Xu J. Tetrahedron: Asymmetry. 2002;13:1129.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

