Comparison of transcriptional responses in liver tissue and primary hepatocyte cell cultures after exposure to hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine
- PMID: 17217515
- PMCID: PMC1780121
- DOI: 10.1186/1471-2105-7-S4-S22
Comparison of transcriptional responses in liver tissue and primary hepatocyte cell cultures after exposure to hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine
Abstract
Background: Cell culture systems are useful in studying toxicological effects of chemicals such as Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), however little is known as to how accurately isolated cells reflect responses of intact organs. In this work, we compare transcriptional responses in livers of Sprague-Dawley rats and primary hepatocyte cells after exposure to RDX to determine how faithfully the in vitro model system reflects in vivo responses.
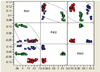
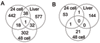
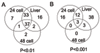
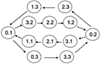
Results: Expression patterns were found to be markedly different between liver tissue and primary cell cultures before exposure to RDX. Liver gene expression was enriched in processes important in toxicology such as metabolism of amino acids, lipids, aromatic compounds, and drugs when compared to cells. Transcriptional responses in cells exposed to 7.5, 15, or 30 mg/L RDX for 24 and 48 hours were different from those of livers isolated from rats 24 hours after exposure to 12, 24, or 48 mg/Kg RDX. Most of the differentially expressed genes identified across conditions and treatments could be attributed to differences between cells and tissue. Some similarity was observed in RDX effects on gene expression between tissue and cells, but also significant differences that appear to reflect the state of the cell or tissue examined.
Conclusion: Liver tissue and primary cells express different suites of genes that suggest they have fundamental differences in their cell physiology. Expression effects related to RDX exposure in cells reflected a fraction of liver responses indicating that care must be taken in extrapolating from primary cells to whole animal organ toxicity effects.
Figures



Similar articles
-
Global gene expression in rat brain and liver after oral exposure to the explosive hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX).Chem Res Toxicol. 2009 Apr;22(4):620-5. doi: 10.1021/tx800444k. Chem Res Toxicol. 2009. PMID: 19239275
-
Up-and-down procedure (UDP) determinations of acute oral toxicity of nitroso degradation products of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX).J Appl Toxicol. 2005 Sep-Oct;25(5):427-34. doi: 10.1002/jat.1090. J Appl Toxicol. 2005. PMID: 16092083
-
Delayed myelosuppression with acute exposure to hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and environmental degradation product hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine (MNX) in rats.Toxicol Appl Pharmacol. 2013 Feb 1;266(3):443-51. doi: 10.1016/j.taap.2012.11.022. Epub 2012 Dec 4. Toxicol Appl Pharmacol. 2013. PMID: 23219714
-
Assessing the non-cancer risk for RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine) using physiologically based pharmacokinetic (PBPK) modeling.Regul Toxicol Pharmacol. 2012 Feb;62(1):107-14. doi: 10.1016/j.yrtph.2011.12.007. Epub 2011 Dec 14. Regul Toxicol Pharmacol. 2012. PMID: 22197625 Review.
-
Water quality criteria for hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX).Regul Toxicol Pharmacol. 1989 Apr;9(2):147-57. doi: 10.1016/0273-2300(89)90032-9. Regul Toxicol Pharmacol. 1989. PMID: 2655037 Review.
Cited by
-
Development of computations in bioscience and bioinformatics and its application: review of the Symposium of Computations in Bioinformatics and Bioscience (SCBB06).BMC Bioinformatics. 2006 Dec 12;7 Suppl 4(Suppl 4):S1. doi: 10.1186/1471-2105-7-S4-S1. BMC Bioinformatics. 2006. PMID: 17217501 Free PMC article.
-
Hepatocyte differentiation.Methods Mol Biol. 2010;640:115-38. doi: 10.1007/978-1-60761-688-7_6. Methods Mol Biol. 2010. PMID: 20645049 Free PMC article.
-
Amino Acid Hepatotoxicity Biomarkers in Human Hepatic Organoids: Promising Standardization of Drug Toxicity Evaluation.ACS Pharmacol Transl Sci. 2025 Jan 4;8(2):510-521. doi: 10.1021/acsptsci.4c00612. eCollection 2025 Feb 14. ACS Pharmacol Transl Sci. 2025. PMID: 39974651
-
A global approach to analysis and interpretation of metabolic data for plant natural product discovery.Nat Prod Rep. 2013 Apr;30(4):565-83. doi: 10.1039/c3np20111b. Nat Prod Rep. 2013. PMID: 23447050 Free PMC article. Review.
-
Metabolic alterations and vulnerabilities in hepatocellular carcinoma.Gastroenterol Rep (Oxf). 2020 Nov 18;9(1):1-13. doi: 10.1093/gastro/goaa066. eCollection 2021 Jan. Gastroenterol Rep (Oxf). 2020. PMID: 33747521 Free PMC article. Review.
References
-
- Layton D, Mallon B, Mitchell W, Hall L, Fish R, Perry L, Snyder G, Bogen K, Malloch W, Ham C, Dowd P. Report No 83, Lawrence Livermore National Laboratory. Livermore, CA; 1987. Data base assessment of the health and environmental effects of conventional weapons demilitarization: Explosives and their co-contaminants.
-
- ATSDR (Agency for Toxic Substances and Disease Registry) US Department of Health and Human Services. Atlanta, GA; 1995. Toxicological Profile for RDX.
-
- US EPA (Environmental Protection Agency) Drinking Water Contaminant Candidate List. Office of Water, US EPA; 2002. http://www.epa.gov/OGWDW/ccl/cclfs.html#table1
-
- Woody RC, Kearns GL, Brewster MA, Turley CP, Sharp GB, Lake RS. The neurotoxicity of cyclotrimethylenetrinitramine (RDX) in a child; a clinical and pharmacokinetic evaluation. J Toxicol Clin Toxicol. 1986;24:305–319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

