Specification of primordial germ cells in medaka (Oryzias latipes)
- PMID: 17217535
- PMCID: PMC1781431
- DOI: 10.1186/1471-213X-7-3
Specification of primordial germ cells in medaka (Oryzias latipes)
Abstract
Background: Primordial germ cells (PGCs) give rise to gametes that are responsible for the development of a new organism in the next generation. Two modes of germ line specification have been described: the inheritance of asymmetrically-localized maternally provided cytoplasmic determinants and the induction of the PGC fate by other cell types. PGCs specification in zebrafish appears to depend on inheritance of germ plasm in which several RNA molecules such as vasa and nanos reside. Whether the specification mode of PGCs found in zebrafish is general for other fish species was brought into question upon analysis of olvas expression--the vasa homologue in another teleost, medaka (Oryzias latipes). Here, in contrast to the findings in zebrafish, the PGCs are found in a predictable position relative to a somatic structure, the embryonic shield. This finding, coupled with the fact that vasa mRNA, which is localized to the germ plasm of zebrafish but does not label a similar structure in medaka opened the possibility of fundamentally different mechanisms governing PGC specification in these two fish species.
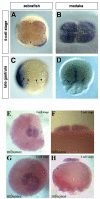
Results: In this study we addressed the question concerning the mode of PGC specification in medaka using embryological experiments, analysis of RNA stability in the PGCs and electron microscopy observations. Dramatic alterations in the somatic environment, i.e. induction of a secondary axis or mesoderm formation alteration, did not affect the PGC number. Furthermore, the PGCs of medaka are capable of protecting specific RNA molecules from degradation and could therefore exhibit a specific mRNA expression pattern controlled by posttrancriptional mechanisms. Subsequent analysis of 4-cell stage medaka embryos using electron microscopy revealed germ plasm-like structures located at a region corresponding to that of zebrafish germ plasm.
Conclusion: Taken together, these results are consistent with the idea that in medaka the inheritance of maternally provided asymmetrically-localized cytoplasmic determinants directs cells to assume the germ line fate similar to zebrafish PGCs.
Figures


Similar articles
-
Dnd is required for primordial germ cell specification in Oryzias celebensis.Gene. 2018 Dec 30;679:36-43. doi: 10.1016/j.gene.2018.08.068. Epub 2018 Aug 30. Gene. 2018. PMID: 30171940
-
Mutations affecting early distribution of primordial germ cells in Medaka (Oryzias latipes) embryo.Mech Dev. 2004 Jul;121(7-8):817-28. doi: 10.1016/j.mod.2004.03.022. Mech Dev. 2004. PMID: 15210188
-
Visualization of Turbot (Scophthalmus maximus) Primordial Germ Cells in vivo Using Fluorescent Protein Mediated by the 3' Untranslated Region of nanos3 or vasa Gene.Mar Biotechnol (NY). 2019 Oct;21(5):671-682. doi: 10.1007/s10126-019-09911-z. Epub 2019 Sep 9. Mar Biotechnol (NY). 2019. PMID: 31502176
-
Germ plasm and molecular determinants of germ cell fate.Curr Top Dev Biol. 2000;50:155-81. doi: 10.1016/s0070-2153(00)50008-8. Curr Top Dev Biol. 2000. PMID: 10948454 Review.
-
Germ line development in fishes.Int J Dev Biol. 1999;43(7):745-60. Int J Dev Biol. 1999. PMID: 10668983 Review.
Cited by
-
Primordial Germ Cell Development in the Poeciliid, Gambusia holbrooki, Reveals Shared Features Between Lecithotrophs and Matrotrophs.Front Cell Dev Biol. 2022 Mar 1;10:793498. doi: 10.3389/fcell.2022.793498. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35300414 Free PMC article.
-
Repressible Transgenic Sterilization in Channel Catfish, Ictalurus punctatus, by Knockdown of Primordial Germ Cell Genes with Copper-Sensitive Constructs.Mar Biotechnol (NY). 2018 Jun;20(3):324-342. doi: 10.1007/s10126-018-9819-3. Epub 2018 Apr 20. Mar Biotechnol (NY). 2018. PMID: 29679251
-
Primordial Germ Cell Specification in Vertebrate Embryos: Phylogenetic Distribution and Conserved Molecular Features of Preformation and Induction.Front Cell Dev Biol. 2021 Sep 16;9:730332. doi: 10.3389/fcell.2021.730332. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34604230 Free PMC article. Review.
-
Perchlorate Exposure Reduces Primordial Germ Cell Number in Female Threespine Stickleback.PLoS One. 2016 Jul 6;11(7):e0157792. doi: 10.1371/journal.pone.0157792. eCollection 2016. PLoS One. 2016. PMID: 27383240 Free PMC article.
-
Fish stem cell cultures.Int J Biol Sci. 2011 Apr 13;7(4):392-402. doi: 10.7150/ijbs.7.392. Int J Biol Sci. 2011. PMID: 21547056 Free PMC article. Review.
References
-
- Rongo C, Broihier HT, Moore L, Van Doren M, Forbes A, Lehmann R. Germ plasm assembly and germ cell migration in Drosophila. Cold Spring Harb Symp Quant Biol. 1997;62:1–11. - PubMed
-
- Seydoux G, Strome S. Launching the germline in Caenorhabditis elegans: regulation of gene expression in early germ cells. Development. 1999;126:3275–3283. - PubMed
-
- Houston DW, King ML. Germ plasm and molecular determinants of germ cell fate. Curr Top Dev Biol. 2000;50:155–181. - PubMed
-
- Hashimoto Y, Maegawa S, Nagai T, Yamaha E, Suzuki H, Yasuda K, Inoue K. Localized maternal factors are required for zebrafish germ cell formation. Dev Biol. 2004;268:152–161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

